Uses of Bisphenol S
Chemical properties
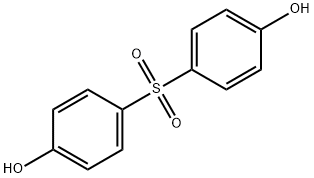
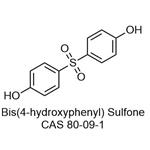
Bisphenol S is white needle crystals with the melting point of T 240-241 ° C. Easily soluble in aliphatic hydrocarbons, soluble in alcohols and ethers, slightly soluble in aromatic hydrocarbons, insoluble in water. Bisphenol S consists of two hydroxyphenyl groups connected by a sulfonyl group with strong electron absorption, so it has stronger acidity than that of other phenols.
Uses
Bisphenol S is mainly used as a fixing agent. In addition, it can be used as a plating solution additive, a leather tanning agent, a dispersant for high-temperature dyeing, Phenol formaldehyde hardener and flame retardant(resins) and the like. It is also an intermediate for pesticides, dyes and additives. As a substitute for bisphenol A, it can be used as a raw material for polycarbonate, epoxy resin, polyester, phenolic resin, and a raw material of polysulfone and polyethersulfone. The product is also used in the manufacture of color photographic materials, contrast aids, thermosensitive recording materials (colour developing reagent), daily surfactants, and high-efficiency deodorants. Fixing agent A ( trade name outside China: Cibatex PA) can be obtained by using bisphenol S as a raw material.
Bisphenol S can be used as a monomer in the synthesis of polysulfone resin, and can also be directly used in coatings, leather modifiers, dye intermediates and metal plating brighteners etc.
Bisphenol S has excellent properties of heat resistance, light resistance and oxidation resistance, and is a raw material for the synthesis of polycarbonate, epoxy resin and polysulfone and polyether oxime resin. It is also used to synthesize dye fixing agent and leather tanning agent, and can also be used as intermediates for metal plating additives, dyes, medicines, pesticides and additives.
Reference
R. L. Melnick, K. A. Thayer, and J. R. Bucher, Conflicting views on chemical carcinogenesis arising from the design and evaluation of rodent carcinogenicity studies. Environ. Health Perspect. 116(1), 130–135 (2008).
90. I. Berenblum, The mechanism of carcinogenesis: a study of the significance of cocarcinogenic action and related phenomena. Cancer Res. 1, 807–814 (1941).
91. P. N. Magee and J. M. Barnes, The production of malignant primary hepatic tumors in the rat by feeding dimethylnitrosamine. Br. J. Cancer 10, 114–122 (1956).
Related articles And Qustion
See also
Lastest Price from Bis(4-hydroxyphenyl) Sulfone manufacturers

US $0.00/KG2025-04-21
- CAS:
- 80-09-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $0.00-0.00/kg2025-04-21
- CAS:
- 80-09-1
- Min. Order:
- 1kg
- Purity:
- 99%+
- Supply Ability:
- 10000kgs per Month