Bis(4-hydroxyphenyl) Sulfone: unveiling the toxic effects and reproductive disruptions of a common compound
General Description
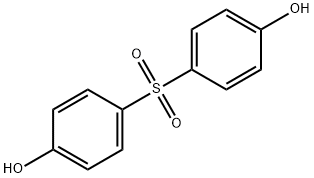
Recent scientific research has shed light on the toxic effects and reproductive disruptions caused by a commonly used compound, bis(4-hydroxyphenyl) sulfone. This novel study reveals that when cells are exposed to bis(4-hydroxyphenyl) sulfone at concentrations similar to human serum levels, it exerts its toxic effects through the induction of oxidative stress and the initiation of inflammatory responses. Further analysis indicates that the compound enhances the production of reactive oxygen/nitrogen species, decreases the levels of antioxidant enzymes, and activates the NLRP3 inflammasome, TLR4, and MAPK pathways to induce inflammatory responses. Additionally, partial alleviation of bis(4-hydroxyphenyl) sulfone-induced toxicity was observed with the administration of the antioxidant N-acetylcysteine (NAC). These findings highlight the necessity to further investigate and remain vigilant about the potential adverse effects associated with bis(4-hydroxyphenyl) sulfone in order to safeguard public health.

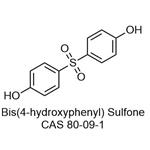
Figure 1. Bis(4-hydroxyphenyl) sulfone
![Article illustration]()
![Article illustration]() Mechanism of action
Mechanism of action
Bis(4-hydroxyphenyl) sulfone was found to exhibit toxic effects through various mechanisms by using murine macrophages. When cells were exposed to bis(4-hydroxyphenyl) sulfone at a concentration of 10-8 M, it induced oxidative stress by increasing the production of reactive oxygen species/reactive nitrogen species (ROS/RNS). This was accompanied by an increase in the levels of oxidant enzymes NOX1/2 and a decrease in the levels of antioxidant enzymes such as SOD1/2, CAT, and GSH-Px. Moreover, exposure to 10-8 M bis(4-hydroxyphenyl) sulfone resulted in the significant production of proinflammatory mediators. This process involved the activation of the NLRP3 inflammasome, TLR4, and MAPK pathways. Interestingly, pretreatment with N-Acetylcysteine (NAC), an antioxidant, mitigated the effects induced by bis(4-hydroxyphenyl) sulfone exposure. Taken together, these findings indicate that bis(4-hydroxyphenyl) sulfone, at concentrations relevant to human serum levels, exerts toxicity by inducing oxidative stress and triggering inflammatory responses in macrophages. It is essential to further investigate and address the potential adverse effects associated with bis(4-hydroxyphenyl) sulfone exposure. 1
![Article illustration]() Toxicology
Toxicology
In vivo toxicity
Recent studies have shown that the exposure to bis(4-hydroxyphenyl) sulfone has been associated with reproductive toxicity in various organisms. In Daphnia magna, an in vivo study reported acute toxicity caused by bis(4-hydroxyphenyl) sulfone exposure. In rats, postnatal bis(4-hydroxyphenyl) sulfone exposure was found to induce uterine growth, which is a marker of estrogen exposure, at both low and high doses. The study also revealed that bis(4-hydroxyphenyl) sulfone had a relative binding affinity of 0.0055% to the nuclear estrogen receptor (ER). A study was conducted using zebrafish (Danio rerio) and observed that bis(4-hydroxyphenyl) sulfone exposure led to decreases in gonad weight, alterations in plasma estrogen and testosterone levels, and disrupted reproduction. This disruption included decreased egg production and hatchability, increased time to hatch, and increased embryo malformations. Another study with zebrafish demonstrated that bis(4-hydroxyphenyl) sulfone exposure resulted in an increased female to male sex ratio, decreased body length, altered concentrations of testosterone, estradiol, and vitellogenin, as well as reproductive disruption. The disruption included decreased egg production, increased time to hatch, and decreased sperm count. Overall, these in vivo studies suggest that exposure to bis(4-hydroxyphenyl) sulfone can have adverse effects on reproductive health in different species, including reduced fertility, hormonal disruptions, and developmental abnormalities. 2
In vivo toxicity
According to several in vitro studies, bis(4-hydroxyphenyl) sulfone exhibits estrogenic activity based on its interaction with human ERα and G-protein coupled receptor 30 (GPR30), consistent response across different cell lines, and physiologically relevant concentrations assessed. Some of these studies suggest that BPS has weaker estrogenic potency than estradiol (E2) in nuclear receptor models. However, in membrane receptor models, bis(4-hydroxyphenyl) sulfone has been shown to have equivalent or greater estrogenic potency compared to E2, activating pathways typically up-regulated by E2. Binding assays indicate that bis(4-hydroxyphenyl) sulfone can bind to the estrogen receptor. Additionally, one study indicates androgenic activity of BPS, while another suggests antiandrogenic activity. bis(4-hydroxyphenyl) sulfone exposure has also been found to induce caspase 8 production, affecting apoptotic and survival signaling, and have effects on hepatic cells, serum albumins binding, and causing DNA damage. 3
Reference
1. Xie C, Ge M, Jin J, et al. Mechanism investigation on Bisphenol S-induced oxidative stress and inflammation in murine RAW264.7 cells: The role of NLRP3 inflammasome, TLR4, Nrf2 and MAPK. J Hazard Mater, 2020, 394:122549.
2. Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect, 2015, 123(7):643-650.
3. Fic A, Egura B, Sollner Dolenc M, Filipi M, Peterlin Mai L. Mutagenicity and DNA damage of bisphenol A and its structural analogues in HepG2 cells. Arh Hig Rada Toksikol, 2013, 64(2):189-200.
Related articles And Qustion
Lastest Price from Bis(4-hydroxyphenyl) Sulfone manufacturers

US $0.00/KG2025-04-21
- CAS:
- 80-09-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $0.00-0.00/kg2025-04-21
- CAS:
- 80-09-1
- Min. Order:
- 1kg
- Purity:
- 99%+
- Supply Ability:
- 10000kgs per Month