Uses and Preparation of Dodecanedioic acid
Dodecanedioic acid (DDDA, C12H22O4, CAS registry No. 693-23-2) has achieved industrial importance in the manufacturing of polyamides, polyesters, lubricating oils and plastic. Dodecanedioic acid (DDDA), a long-chain dicarboxylic acid, generally represented as HOOC-(R)n-COOH, has become one of the commonly used monomers for the synthesis of aliphatic long-chain polyesters(ALCPEs) due to its commercial availability. DDDA homologues and the metabolites are extremely difficult to be separated from aqueous solution because the extreme pooraqueous solubility of DDDA[1]and homologues leads to uncon-trolled crystallization process.
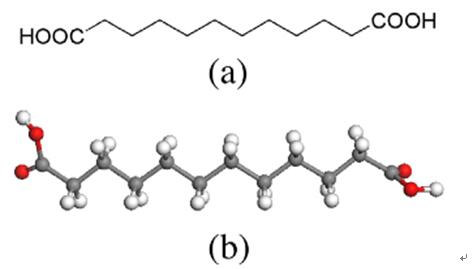
Fig 1. Chemical structure formula (a) and three-dimensional structure (b) of DDDA
Dodecanedioic acid is a dicarboxylic acid. DDDA is a white solid with slight odor. Its melting point is 1280℃ and boiling point is 2500℃. DDDA is used as adhesives, capacitor electrolyte and resistant coats. There is no health issue associated with inhalation of DDDA due to its lower vapor pressure, and DDDA is neither genotoxic nor mutagenic. Dodecanedioic acid can be produced from microbial fermentation of vegetable oils, such as vernonia galamensis oil[2], palm kernel oil and coconut oil[3].Compared with short-chain aliphatic acids, DA is more suitable for the preparation of (co)polyesters, as short-chain aliphatic acids tend to stimulate intramolecular condensation[4]. Dodecanedioic acid is condensed with hexamethylene diamine to obtain engineering plastic nylon 6-12[5]; a diester formed with an alcohol such as octanol or butanol, which can be used as an increase in polyvinyl chloride, nitrocellulose or cellulose acetate. Plasticizers, diesters synthesized with octanol are also used as high-grade lubricants for jet engines and gas turbines, or base oils for low-temperature greases; DDDA can also be used as a modifier for saturated polyesters, precipitation of metals And raw materials for spices and special polyurethanes[6].
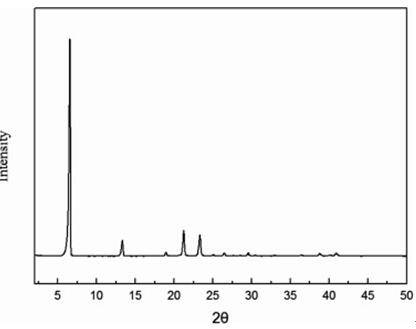
Fig 2. Powder X-ray diffraction pattern for DDDA
Industrially, butadiene can be trimerized to obtain cyclododecatriene, which is then hydrogenated to form cyclododecane, which is then oxidized with nitric acid to obtain dodecanoic acid. Alternatively, the cyclohexane can be used as a raw material, and reacted with hydrogen peroxide in methanol to prepare an alkoxycyclohexyl peroxide, which is then ring-opened and dimerized to form methyl dodecanoate. After saponification, DDDA can be obtained. In the laboratory preparation, cyclododecanol was used as a raw material, and nitric acid was obtained by oxidation with nitric acid.
Dodecanedioic acid is a dicarboxylic acid which is water soluble and involves in a metabolic pathway intermediate to those of lipids and carbohydrates[7]. Dodecanedioid acid is an indicator of hepatic carnitine palmitoyltransferase I (CPT IA) deficiency. CPT IA deficiency is characterized by hypoketotic dicarboxylic aciduria with high urinary levels of dodecanedioic acid. This DDDA dicarboxylic aciduria suggests that carnitine palmitoyltransferase I may play a role in the uptake of long-chain dicarboxylic acids by mitochondria after their initial shortening by beta-oxidation in peroxisomes[8].
Dodecanedioic acid infusion decreases plasma glucose levels in NIDDM patients to normal range without influencing plasma insulin levels. The balance between pyruvate and lactate was affected by DDDA infusion only in diabetics patients. DDDA might represent a fuel substrate immediately available for tissue energy requirements, especially in conditions such as diabetes mellitus in which glucose metabolism is impaired.
References
[1] Zakharkin L. I., Vinogradova L. P., Kotneva V. V.et al. The synthesis of brassylic and 1,12-dodecanedicarboxylic acids[J]. Bulletin of the Academy of Sciences of the Ussr Division of Chemical Science. 1962,11(7):1230-1232.
[2] Zhou Changfeng, Wei Zhiyong. Biobased long-chain aliphatic polyesters of 1,12-dodecanedioic acid with a variety of diols: Odd-even effect and mechanical properties[J]. Materialstoday Communication. 2019,19(3):450-458.
[3] Celli Annamaria, Barbiroli Giancarlo, Berti Corradoet al. Thermal properties of poly(alkylene dicarboxylate)s derived from 1,12‐dodecanedioic acid and even aliphatic diols[J]. Journal of Polymer Science Part B. 2010,45(9):1053-1067.
[4] Papageorgiou George Z., Bikiaris Dimitrios N., Achilias Dimitris S.et al. Synthesis and comparative study of biodegradable poly(alkylene sebacate)s[J]. Journal of Polymer Science Part B Polymer Physics. 2010,48(6):672-686.
You may like
Related articles And Qustion
See also
Lastest Price from Dodecanedioic acid manufacturers
US $0.00/kg2025-10-28
- CAS:
- 693-23-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $1.00-4.00/KG2025-09-11
- CAS:
- 693-23-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG