Azelaic Acid: A Versatile Chemical Compound in Modern Applications
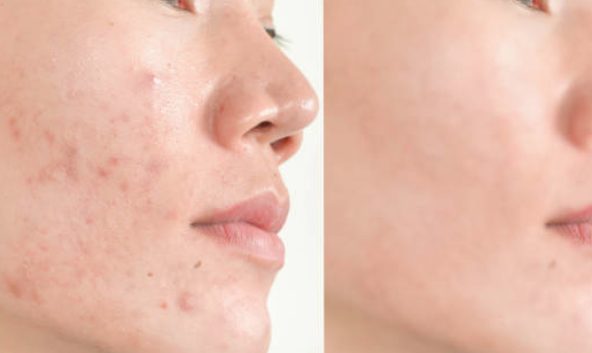
Azelaic acid is commonly used for acne vulgaris, rosacea (rosacea), and perioral dermatitis. It has a certain effect on post-inflammatory pigmentation (such as acne marks, etc.) and melasma. It can also be used for seborrheic dermatitis associated with rosacea. Azelaic acid can also be used to treat rosacea, and has a place in the maintenance treatment of acute papule-pustular stage and stable stage.
As a non-antibiotic topical acne treatment drug, azelaic acid can be used for a long time without drug resistance. It is a category B drug for pregnancy and is relatively safe. Azelaic acid is well tolerated, but some patients will experience irritation reactions after use, such as erythema, burning, desquamation, itching, etc., most of which are temporary.

How to use azelaic acid?
Apply azelaic acid after cleansing, gently massage until absorbed, and wash off after 5 minutes. It is recommended to establish tolerance for the first use, try it on a small scale, apply a small amount of product on the forearm or behind the ear, and observe whether there is irritation or allergic reaction. After about 3-7 consecutive days of tolerance, the retention time and dosage of azelaic acid can be increased. After tolerance is established, apply it for half an hour and wash off the azelaic acid.
1) Use during the day, the face is not greasy, you can wash your face with clean water, and directly apply an appropriate amount of 20% azelaic acid. If you feel dry, you can continue to apply moisturizing products after applying azelaic acid, and then apply sunscreen.
2) Use at night, after cleaning the face, still directly apply 20% azelaic acid. If you feel dry, you can add skin care products later.
Key points: Azelaic acid is the first step after washing the face, and it is used before all skin care products. The reason for doing this is to meet the drug needs of acne treatment first, and then meet the needs of skin care. In addition, where there are pustules, apply clindamycin phosphate gel 3 times a day until it subsides.
Precautions
1) Azelaic acid has almost the common adverse reactions of acid: redness, itching, dryness, etc. It is recommended to use low concentrations, small areas, and small amounts for the first time. Those who are intolerant should use SCT therapy to gradually establish tolerance. When using azelaic acid, pay attention to skin moisturizing and sun protection.
2) If you don't have skin problems and are only using it for oil control and keratin metabolism, there's no need to use high-concentration azelaic acid too frequently. It's similar to an acid brush, control the pace yourself, and don't pursue shedding a layer of skin.
3) After using azelaic acid, be sure to immediately wash the hands that applied the product to your face, whether it's skin care or medicine. After washing your hands, use the same hand to care for your skin or rub your eyes.
Related articles And Qustion
Lastest Price from Azelaic acid manufacturers

US $0.00-0.00/kg2025-09-28
- CAS:
- 123-99-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 1000kgs

US $0.00/KG2025-08-30
- CAS:
- 123-99-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000KG