Applications of 3-bromodibenzo[b,d]thiophene
3-bromodibenzo[b,d]thiophene is an important organic intermediate to synthetize substituted dibenzo[b,d]thiophene products.
Applications
The following example is about its application on the synthesis of organic electroluminescent device [1]
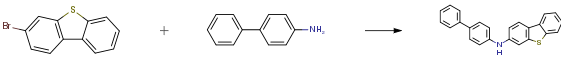
3-bromodibenzothiophene (12.0 g, 45.6 mmol),4-aminobiphenyl (8.5 g, 50.2 mmol), tris(dibenzylideneacetone)dipalladium (0.4 g, 0.5 mmol), 2-dicyclohexylphosphino-2,4,6-triisopropylbiphenyl (0.4g, 0.9mmol) and sodium tert-butoxide (6.6g, 68.4mmol) were added to toluene (120mL), heated to 105-110 ° C under nitrogen atmosphere, stirred for 1.5 hours; cooled to room temperature, the reaction solution was washed twice with water After drying with magnesium sulfate, the filtrate was filtered through a short silica gel column, and the solvent was removed under reduced pressure. The crude product was recrystallized and purified using a dichloromethane/ethanol system. Obtained as a light brown solid (13.9 g, 87%).
The following example is about its application on the synthesis of 2,4-thiadiazole compounds [2]

In a 500mL three-necked bottle,(9H-carbazol-3-yl)boronic acid (25.32 g, 120 mmol),3-bromodibenzothiophene (31.58 g, 120 mmol),Sodium tert-butoxide (23.04 g, 240 mmol),Tri-tert-butylphosphine tetrafluoroborate (0.21 g, 0.72 mmol), added 250 mL of toluene, Tris(dibenzylideneacetone)dipalladium (0.33 g, 0.36 mmol) was added under a nitrogen atmosphere. The temperature was raised to 115 ° C for 16 h, the liquid phase monitoring reaction was completed, cooled to room temperature, and washed with water.Filtration, column chromatography, To obtain (9- (dibenzothiophen-3-yl) -9H- carbazol-3-yl) boronic acid 38.70 g, yield 82 percent
The following example is about its application on the synthesis of aniline derivatives [3]

Into the flask, N1-(4-((4-((4-(diphenylamino)phenyl)amino)phenyl)amino)phenyl)-N4,N4-diphenylbenzene-1,4-diamine 2.00 g, 2-bromodibenzo[b, d] thiophene 2.79g, Pd (dba) 2 50.3mg, and was placed in a t-butoxy sodium 1.21g, the inside of the flask was replaced with nitrogen. Thereto, was placed toluene 20mL and previously prepared toluene solution of tri-t- butyl phosphine was allowed 0.750 mL (concentration 47.2 g / L), and stirred at 50 ° C 7 hours. After the stirring, the reaction mixture was cooled to room temperature and the reaction mixture was cooled, toluene, liquid separation process by mixing the saturated brine. The resulting organic layer was dried over sodium sulfate, then stirred at room temperature for 30 minutes the active carbon 0.2g was added to the dried organic layer. Thereafter, removing the active carbon by filtration, the solvent was distilled off under reduced pressure from the filtrate, the resulting residue was dissolved in toluene 20 m. The resulting solution was added dropwise to a mixed solvent (300mL / 200mL) in methanol and ethyl acetate, and stirred the resulting slurry at room temperature and then recovered by filtration was filtered slurry. Then, the resulting filtrate was used a solution prepared by dissolving in toluene 20 mL, the operations up recovery of dripping Kararoof the mixed solvent were carried out in the same manner as described above. Finally, the recovered filtrate was dried, to give the product (yield 3.23 g, 90percent yield).
The following example is about its application on the synthesis of pyrimidine derivative [4]

3-bromodibenzothiophene (1 eq) in a 250 ml three-necked flask, added to a dry tetrahydrofuran solution, and cooled to -78 ° C. After adding n-butyllithium (2.5M, 1.1 eq), the reaction was kept at -78 °C for 1.5 h. Triisopropyl borate (ρ = 0.815 g / ml, 1.1 eq) was added dropwise, and after completion of the dropwise addition, the temperature was raised to room temperature. After 8 hours, the reaction was quenched with dilute hydrochloric acid, and then distilled to remove tetrahydrofuran under reduced pressure. Added dissolved in dichloromethane, washed with water, dried over anhydrous magnesium, sulfate, filtered, and the filtrate was recrystallized, to give the product as a white solid (90 percent).
References
1.Shanxi Laite Optoelectric Materials Co., Ltd. Ma T, Feng Z, Yang L, Li H, Sha X. Aromatic derivative containing polycyclic alkane and organic electroluminescent device containing the derivative (by machine translation). CN110183332[P], 2019, A, Paragraph 0134; 0135.
2.Wuhan Shang Sai Optoelectric Technology Co., Ltd. Mu G, Zhuang S, Ren C. 2, 4 - Thiadiazole compounds and its preparation and use (by machine translation). CN109232474[P], 2019, A, Paragraph 0153-0155.
3.Nissan Chemical Industrieslimited. Nakaie N, Nakazawa T. The use and imidating (by machine translation). JP5790901[P], 2015, B1, Paragraph 0247; 0248
4.Huazhong University of Science and Technology. Wang L, Zhang Q, Zhuang S. A pyrimidine derivatives and application thereof (by machine translation). CN108285452[P], 2018, A, Paragraph 0035; 0047; 0048; 0049.
You may like
Related articles And Qustion
See also
Lastest Price from 3-bromodibenzo[b,d]thiophene manufacturers
![97511-04-1 3-bromodibenzo[b,d]thiophene](/ProductImageEN/2024-03/Small/2ca0d49a-31dc-4079-be8c-a29b6c3aab5f.gif)
US $0.00/kg2025-04-21
- CAS:
- 97511-04-1
- Min. Order:
- 1kg
- Purity:
- 99.5% HPLC
- Supply Ability:
- 10 tons
![97511-04-1 3-bromodibenzo[b,d]thiophene](/ProductImageEN/2023-12/Small/dc96bbb9-3c07-4f40-a61c-87edf674e15b.png)
US $1.00/kg2024-11-07
- CAS:
- 97511-04-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20000
![97511-04-1 3-bromodibenzo[b,d]thiopheneapplication on the synthesis](https://www.chemicalbook.com/CAS/20200331/GIF/97511-04-1.gif)
