3-Bromodibenzo[b,d]thiophene: properties, applications and safety
General Description
3-Bromodibenzo[b,d]thiophene is a chemical compound with unique properties including reactivity, thermal stability, and desirable electronic characteristics. It finds applications in various fields such as organic synthesis and optoelectronics. It serves as a precursor for the synthesis of polymers with narrow molecular weight distribution and predictable molecular weight. Additionally, it can be used for the synthesis of novel monomers and preparation of polymers with dibenzothiophenium salt moieties. However, caution should be exercised when handling this compound due to its potential skin and respiratory irritation, as well as its harmful effects on aquatic life. Proper safety measures and disposal procedures should be followed.
![Figure 1. 3-Bromodibenzo[b,d]thiophene.png Article illustration](/NewsImg/2023-11-27/6383668572844128165945388.jpg)
Figure 1. 3-Bromodibenzo[b,d]thiophene
Properties
3-Bromodibenzo[b,d]thiophene is a chemical compound belonging to the class of brominated dibenzothiophenes. It is characterized by its unique properties and finds applications in various fields. Firstly, 3-Bromodibenzo[b,d]thiophene possesses a distinctive molecular structure with a bromine atom attached to the benzene ring of the dibenzothiophene backbone. This bromine substitution enhances its reactivity, allowing it to participate in various chemical reactions. Secondly, this compound exhibits good thermal stability, making it suitable for high-temperature applications. It can withstand elevated temperatures without undergoing significant decomposition or degradation, which is advantageous in industries requiring stable materials. Furthermore, 3-Bromodibenzo[b,d]thiophene displays notable electronic properties. Due to its conjugated system, it exhibits strong absorption in the ultraviolet-visible region, making it potentially useful in optoelectronic devices such as organic light-emitting diodes (OLEDs) and photovoltaic cells. In summary, 3-Bromodibenzo[b,d]thiophene possesses unique properties including reactivity, thermal stability, and desirable electronic characteristics. These attributes make it a valuable compound for various applications, ranging from organic synthesis to optoelectronics. 1
Applications
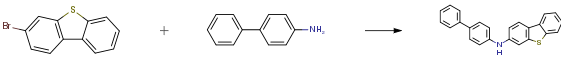
3-Bromodibenzo[b,d]thiophene has shown diverse applications in various fields of chemistry. It serves as a precursor for the synthesis of 2-vinyldibenzothiophene (2VDBT), which can undergo anionic polymerization and copolymerization with styrene. The anionic polymerization of 2VDBT exhibits a living character, resulting in polymers with narrow molecular weight distribution and predictable molecular weight. The homopolymerization of poly(2VDBT) yields high-quality materials, considering the potential overestimation of experimental molecular weight due to the bulky dibenzothiophenyl group. Additionally, block copolymers with narrow polydispersity can be obtained through sequential copolymerization of 2VDBT and styrene. Furthermore, 2BDT can be utilized for the synthesis of 2-(p-vinylbenzyl)dibenzothiophene (1) via a Ni-catalyzed coupling reaction. Homopolymerization and copolymerization of 1 with styrene result in polymers with high yields. Thermal analysis reveals that the copolymers exhibit increased weight-loss and glass transition temperatures with higher content of 1. Moreover, 2BDT can be reacted with CH3I–AgBF4 to form a polymer containing dibenzothiophenium salt moieties (poly(sulfonium salt), 3). This polymer consists of 63 mol% of the methyldibenzothiophenium tetrafluoroborate unit. Thermal decomposition of 3 occurs in two steps, with the lower temperature decomposition likely involving the elimination of methyl tetrafluoroborate moieties. In summary, 3-Bromodibenzo[b,d]thiophene finds applications in anionic polymerization, copolymerization with styrene, synthesis of novel monomers, and preparation of polymers with dibenzothiophenium salt moieties. These applications demonstrate the versatility and potential of 3-Bromodibenzo[b,d]thiophene in various chemical fields. 2,3
Safety
3-Bromodibenzo[b,d]thiophene is a chemical compound that is commonly used in various industrial applications. While this compound has many potential uses, it is important to consider its safety when handling or using it. Studies have shown that 3-Bromodibenzo[b,d]thiophene can cause skin irritation upon contact. Therefore, it is recommended to wear protective gloves and clothing when handling this compound. Additionally, inhalation of the compound's fumes can irritate the respiratory system and lead to coughing, wheezing, and shortness of breath. Proper ventilation is essential when working with this compound to minimize any potential health risks. Furthermore, 3-Bromodibenzo[b,d]thiophene has been found to be harmful to aquatic life. Therefore, it is important to dispose of any unused or waste material properly to avoid contaminating water sources. In conclusion, while 3-Bromodibenzo[b,d]thiophene has many potential uses, it should be handled with caution due to its potential health and environmental risks. 4
Reference
1. PubChem. COMPOUND SUMMARY: 3-bromoDibenzothiophene. National Library of Medicine, PubChem CID: 13415616.
2. Avila-Ortega A, Vázquez-Torres, H. Living anionic polymerization of 2-vinyldibenzothiophene: Homopolymer and block copolymers with styrene. Journal of Polymer Science Part A: Polymer Chemistry, 2007, 45(10): 1993-2003.
3. Osamu S. Synthesis radical polymerization 2-vinyldibenzothiophene. Journal of Polymer Science Part A: Polymer Chemistry, 2000, 35(14): 2813–2819.
4. 3-Bromodibenzo[b,d]thiophene. European Chemicals Agency, EC / List no. 815-535-2.
Related articles And Qustion
See also
Lastest Price from 3-bromodibenzo[b,d]thiophene manufacturers
![97511-04-1 3-bromodibenzo[b,d]thiophene](/ProductImageEN/2024-03/Small/2ca0d49a-31dc-4079-be8c-a29b6c3aab5f.gif)
US $0.00/kg2025-04-21
- CAS:
- 97511-04-1
- Min. Order:
- 1kg
- Purity:
- 99.5% HPLC
- Supply Ability:
- 10 tons
![97511-04-1 3-bromodibenzo[b,d]thiophene](/ProductImageEN/2023-12/Small/dc96bbb9-3c07-4f40-a61c-87edf674e15b.png)
US $1.00/kg2024-11-07
- CAS:
- 97511-04-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20000
![97511-04-1 Properties of 3-Bromodibenzo[b,d]thiopheneapplications of 3-Bromodibenzo[b,d]thiophenesafety of 3-Bromodibenzo[b,d]thiophene](https://www.chemicalbook.com/CAS/20200331/GIF/97511-04-1.gif)