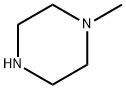
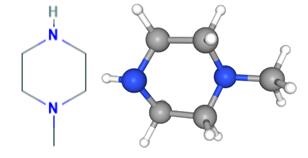
Applications of 1-Methylpiperazine
1-Methylpiperazine is a colorless liquid. The boiling point of 138℃ (140℃) and relative density of 0.903 (20/4℃), 1.4378 refractive index, a flash point of 42℃, soluble in water, ethyl ether, ethanol, water, methanol and other arbitrary than immiscible in water was alkaline.
1-Methylpiperazine is a common building block used in organic synthesis. For example, N-methylpiperazine is used in the manufacture of various pharmaceutical drugs including cyclizine[1], meclizine, and sildenafil.
The lithium salt, lithium 1-Methylpiperazine, is used as a reagent in organic synthesis for protection of aryl aldehydes.
The cyclic diamine 1-Methylpiperazine is a pale yellow oil with a boiling point of 286 ºC. 1-Methylpiperazine can be synthesized in several ways, including treating 1,4-dimethylpiperazine with Raney nickel. In 1998, M. A. Pericàs and coauthors used 1-Methylpiperazine to prepare resin-anchored chiral catalysts. But the best use of 1-Methylpiperazine may be protecting people from mosquito bites. U. R. Bernier and co-workers at the USDA Agricultural Research Service showed that bacteria on human skin form 1-Methylpiperazine and other small heterocycles that prevent mosquitoes from recognizing that someone is present. The researchers recently applied for a patent covering this use for the active compounds.
Industrially, N-methylpiperazine is produced by reacting diethanolamine and methylamine at 250 bar and 200 °C.1-Methylpiperazine is a useful mimic template for preparation of molecularly imprinted microspheres. In addition to this, it is used in the generation of difunctional strong anion-exchange stationary phase from a 1,4-diazacyclohexane derivative[3].
1-Methylpiperazine is prepared by six water by methyl piperazine derived reaction. Six of water and piperazine hydrochloride joined 11:50, heated to 45℃, 71/92 formic acid and formaldehyde in the mixture. Canada finished at 50℃ reaction about 2-3 hours, then returned warming, carbon dioxide gas to escape not far. Cool to 80℃, by adding hydrochloric acid, acid heating steam to dry. Dressed adding methanol and heating return 30min mixture filter (Residue for two piperazine hydrochloride). Filtrate recycling to make methanol, the residue by adding sodium hydroxide solution to pH=14, distillation, in aquifers methyl piperazine. Heating benzene increases with the return of water to do, fractionation, 132-140℃ distillate collection, in anhydrous methyl piperazine. The yield of 1-Methylpiperazine is about 50%.
Toxicity: Poison by intraperitoneal route. Moderately toxic by inhalation, ingestion, and skin contact. A severe skin and eye irritant. Flammable liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx.
References
[1] Vardanyan, Ṛuben & Hruby, Victor J. Synthesis of Essential Drugs. p. 226.
[2] Yan, Hongyuan., et al., 2012. Simultaneous determination of four plant hormones in bananas by molecularly imprinted solid-phase extraction coupled with high performance liquid chromatography. The Analyst. 137(12): 2884-90.
[3] Kovalev, V. V.; Gorbunova, Y. E.; Kokunov, Y. V. Silver nitrate coordination polymer with 1-methylpiperazine: Synthesis and crystal structure. Russ. J. Inorg. Chem. 2015, 60 (7), 886-888.
You may like
Related articles And Qustion
See also
Lastest Price from 1-Methylpiperazine manufacturers

US $10.00/kg2025-04-21
- CAS:
- 109-01-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Bag2025-04-21
- CAS:
- 109-01-3
- Min. Order:
- 2Kg/Bag
- Purity:
- 99% up, High Density
- Supply Ability:
- 20 tons