1-Methylpiperazine: exploring the properties and applications of a versatile organic compound
General Description
1-Methylpiperazine is a chemical compound with interesting properties and diverse applications. It belongs to the piperazine family and is characterized by the presence of a methyl group attached to the piperazine ring. In terms of its physical and chemical properties, 1-Methylpiperazine is a colorless liquid with a distinctive amine odor. It is soluble in water and common organic solvents. This compound exhibits basicity due to the presence of the secondary amine group, which makes it useful in various chemical reactions and as a building block in organic synthesis. One of the notable applications of 1-Methylpiperazine is in the pharmaceutical industry. Its unique structure and reactivity make it a valuable component in the development of new therapeutic compounds. Additionally, 1-Methylpiperazine finds utility as a corrosion inhibitor in industrial settings. It has been studied for its ability to inhibit metal corrosion at elevated temperatures and in harsh environments. The compound demonstrates excellent corrosion inhibitory properties and can be used to protect metals from degradation and extend their lifespan. In summary, 1-Methylpiperazine is a versatile compound with diverse applications. Its role as an intermediate in pharmaceutical synthesis, corrosion inhibitor in industrial processes, and additive in materials science make it an important component in various fields.

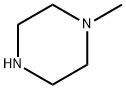
Figure 1. 1-Methylpiperazine
Properties
1-Methylpiperazine is a versatile organic compound with diverse physical and chemical properties. It is a colorless liquid with a distinct amine-like odor. At room temperature, it remains in the liquid state, with a melting point of -18°C and boiling point range of 150-152°C. The density of 1-Methylpiperazine at 25°C measures 0.862 g/cm3. It exhibits good solubility in both water and various organic solvents. Although considered stable under normal conditions, it can react with strong oxidizing agents or acids. As a functional group-containing compound, it acts as a nucleophile, participating in reactions such as alkylation, acylation, and substitution. Understanding these properties is important for its applications in pharmaceuticals, industrial processes, and chemical synthesis. 1
Applications
Pharmaceutical intermediate
1-Methylpiperazine diphenoxy-substituted adamantanes derivative Ia exhibits significant pharmacological activity against T. brucei, the causative agent of sleeping sickness. In comparative studies with other adamantane derivatives containing phenoxyl and aminoalkyl side chains, Ia displayed the highest potency against this parasitic infection. Furthermore, it has demonstrated promise as an antitubercular agent. Among its analogs, hexylamine IIf showed superior antimycobacterial activity. It is worth noting that the inclusion of an ethylene spacer in the side chain led to enhanced efficacy when compared to the 2-hydroxypropylene linker. These encouraging preliminary results pave the way for future investigations into phenyl-substituted adamantane derivatives, aiming to develop potent compounds with trypanocidal and antitubercular properties while maintaining a low level of toxicity towards mammalian cells. 2
Corrosion Inhibitor
Piperazine compounds (1-Methylpiperazine) have been studied for their corrosion inhibition properties, showing attractive advantages such as a low thermal degradation rate up to 150°C, low oxidation rate in the presence of metal ions (Cr, Ni, Fe, and V), and fast adsorption rate. These characteristics make piperazine an appealing alternative solvent to CO2 in corrosion control applications. Piperazine compounds (1-Methylpiperazine) also exhibit potential as chelating agents due to their ability to form complexes with metal ions. However, there have been limited investigations on the corrosion behavior of metals in concentrated piperazine solutions. For instance, sodium silicate has been found to provide maximum corrosion inhibition of 61% at concentrations of 10 and 15 ppm in chloride (Cl-) and sulfate (SO4 2-) ion-containing environments. This can be attributed to the formation of a silica-oxide composite layer, which occurs through donor-acceptor interactions between the inorganic corrosion inhibitor's free electrons and the metal's vacant orbitals, as well as the substitution of water molecules by silica molecules in the double layer. Furthermore, piperazine has shown maximum corrosion inhibition of 76% at a concentration of 2 ppm in chloride (Cl-) and sulfate (SO4 2-) ion-containing environments. The corrosion inhibition is likely due to the film-forming function of piperazine at both the anodic and cathodic sites, although the exact mechanism remains undefined. In optimizing corrosion inhibition, a composite system using a moderately low dosage of corrosion inhibitors has demonstrated tremendous effectiveness. Specifically, 2 ppm of piperazine and 10 ppm of sodium silicate were found to exhibit synergistic effects through independent and silicate bridge adsorption mechanisms. 3
Reference
1. Starosta R, Bazanów B, Barszczewski W. Chalcogenides of the aminomethylphosphines derived from 1-methylpiperazine, 1-ethylpiperazine and morpholine: NMR, DFT and structural studies for determination of electronic and steric properties of the phosphines. Dalton Trans, 2010, 39(32):7547-7555.
2. Georgiadis MO, Kourbeli V, Ioannidou V, et al. Synthesis of diphenoxyadamantane alkylamines with pharmacological interest. Bioorg Med Chem Lett, 2019, 29(11):1278-1281.
3. Ghaffari, S., Aliofkhazraei, M. & Rouhaghdam, A.S. Corrosion Inhibition of Sodium Silicate and Piperazine and Their Synergistic Effect on Carbon Steel in Soft Water Media. Prot Met Phys Chem Surf, 2019, 55:1195–1206.
Related articles And Qustion
See also
Lastest Price from 1-Methylpiperazine manufacturers

US $10.00/kg2025-04-21
- CAS:
- 109-01-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Bag2025-04-21
- CAS:
- 109-01-3
- Min. Order:
- 2Kg/Bag
- Purity:
- 99% up, High Density
- Supply Ability:
- 20 tons