Application, Mechanism of action, and Synthesis of 1,3,5-Triglycidyl isocyanurate
Ethyl valerate (CAS No: 2451-62-9), also known as the green apple flavor is well known for its wide applications in the areas of food, pharmaceuticals and cosmetics industries.Next generation biofuels will have to rely on more abundant and sustainable supply chains. Progresses in biomass processing have made lignocellulose more attractive for the production of liquid biofuel, such as ethyl valerate. Indeed, levulinic acid obtained from lignocellulose can be converted into esters by hydrogenation and esterification. Nowadays, biofuels are used as fuels in engines (spark and diesel engines) in replacement or mixed with traditional fuels as gasoline and diesel [1-4].Pentanoate esters, methyl and ethyl valerate (C6H12O2 and C7H14O2) can be used, as fuels, in a spark engine.
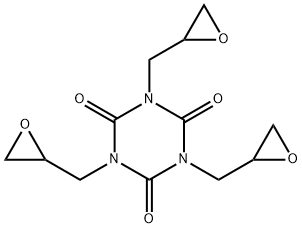
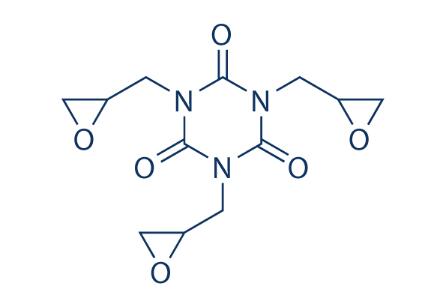
1,3,5-Triglycidyl isocyanurate (Teroxirone) (CAS No: 539-82-2), appears as a while crystalline solid, is a triazene triepoxide with anticancer and antineoplastic activity [1]. Teroxine alkylates and cross-links DNA, thereby inhibiting DNA replication [1]. It is a novel triepoxide, synthesized as an alkylator and showing a broad spectrum of preclinical activity. It is also a crosslinking agent in polymer synthesis; additive in plastic, rubber, and adhesives; hardener in polyester powder coatings; in protective coatings of electronic devices; in top-coated and solder-resistant inks[2].

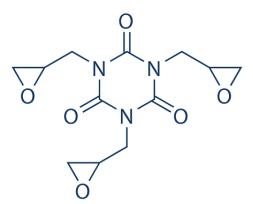
Figure 1 the molecular formula of teroxirone
Application and pharmacokinetics
Triglycidyl isocyanurate has been used as a curing agent for polyester resins in weather-resistant powder coatings in Europe for about 20 years. In Denmark Triglycidyl isocyanurate has an extended use in powder coatings. A powder coating contains up to 10 % Triglycidyl isocyanurate. It is sprayed directly onto metal objects by an electrostatic process. Powder coated objects include office and garden steel furniture, car parts, metal fencing, window and door frames, shelving, electrical equipment, and domestic appliances such as refrigerators, washing machines and ovens. [3]. Triglycidyl isocyanurate is also used in electrical insulation materials, resin moulding systems, laminated sheeting’s, printed circuits, tools, inks, adhesives, lining materials, stabilizers for plastics and amine captures.
It has antiangiogenic and antineoplastic activities. It has been tested clinically and demonstrated effective against tumor growth. The proven efficiency in treating patients offered teroxirone a new hope as an effective antiangiogenic agent [4]. In transplanted models, teroxirone was effective in inhibiting growth of murine tumors without influencing animal survival [5]. The active cytotoxic mechanism involves drug binding to nuclear proteins of cancer cells, thereby leaving the damaged DNA as induced beyond repair and unable to replicate. The mechanism differed from platinum-based anticancer agents that injure cancer cells by interacting with DNA directly [6]. Furthermore, recent studies have shown that triglycidyl isocyanurate inhibits the growth of non-small-cell-lung cancer cells via p53 activation, which induces cell apoptosis. Studies have also found that teroxirone inhibited growth of human hepatocellular carcinoma (HCC) cells, but not hepatic cells. The drug induced apoptosis in HCC cells bearing mutant p53. Pretreatment of caspase-3 inhibitor restored cell viabilities by suppressing extrinsic pathway-mediated cell death. More experiments suggested that sub-apoptotic concentrations of teroxirone mitigated migration, invasion and wound healing of HCC cells. The drug reduced growth of the xenograft tumors as established in animal models by activating apoptotic death.
An old phase I trial of teroxirone [7] has found that it has good cytotoxic activity against sublines of P388 and L1210 leukemias resistant to another alkylating agent, cyclophosphamide. Thirty-six patients received teroxirone as a single iv push for 5 sequential days every 4 weeks. The dose-limiting toxic effects were phlebitis and cutaneous "flare" reactions, with a maximal tolerated dose of 340 mg/m2/day X 5. Nausea, vomiting, and myelosuppression were present but were not dose-limiting at the maximal tolerated dose. This dose would probably be a reasonable dose for phase II trials, but could not be delivered repeatedly without a central line. Since the local reactions were very severe and unique, we believe that studies on the safety of repeated administration via a central line should be completed in animals before trials of systemic therapy in humans begin.
Its pharmacological kinetics is also available in old studies [8]. Following rapid infusion to patients, plasma disappearance is characterized by a one-compartment open model with mean values for t1/2, total body clearance and volume of distribution of 1.4min, 5.7ℓ/min, and 13ℓ, respectively. When it is administered by i.v. infusion (60–240min), plateau plasma concentrations are rapidly achieved, followed by rapid disappearance at the end of infusion. It is rapidly metabolized by hepatic, but not lung, microsomal preparations. Metabolism is NADPH independent and is inhibited by cyclohexene oxide. Epoxide hydrolysis products were detected in microsomal preparations and rabbit urine following administration of it. Its cytotoxicity to human tumor cell lines in culture is abolished in the presence of 9,000 x g rat liver preparations, and is restored by the addition of cyclohexene oxide to incubation mixtures.
Mechanism of action
Teroxirone induces apoptotic cell death of tumor cells. The status of the tumor suppressor p53 determined the onset of apoptotic cell death in human non-small cell lung cancer cells (NSCLC) [9]. Furthermore, studies [9] have found that subsequent to a 12 h treatment with low concentrations of teroxirone, mitochondrial membrane potential (MMP) was suppressed, followed by ROS production and apoptosis in lung cancer cells carrying wild type p53. N-acetylcysteine inhibited apoptotic cell death. The depleted expression of p53, reduction of apoptosis-associated active caspase-3 and poly ADP-ribose polymerase cleavage with resurgence of the pro-survival signal protein kinase B, all demonstrated an antioxidant-mediated reduction of apoptosis by teroxirone. The diminished ROS intensity inhibited the release of mitochondrial cytochrome c and DNA damage. The present study provided evidence that teroxirone treatment induced the ROS-activated intrinsic apoptotic pathway, which led to cell death in human NSCLC cells.

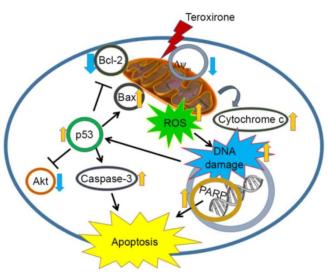
Figure 2 Teroxirone activates p53-mediated induction of apoptosis (Ref 7)
Synthesis
The esterification reaction was performed in a 100 mL screw-capped glass bottle using containing 6 mL of reaction mixture composed by stoichiometric molar ratio of reactants (ethanol and valeric acid) in heptane medium. The reaction mixture was maintained under continuous agitation in an orbital shaker [11,15]. The conversion percentage was determined by measuring the concentration of residual valeric acid after a given reaction time [18–20]. Aliquots (0.1 mL) were periodically withdrawn from the reaction mixture, added to an ethanol/acetone 1:1 (v/v) mixture (10 mL) and titrated with NaOH solution (20 mM) using phenolphthalein as indicator.
References
1. Kim, S. H., et al. "Teroxirone suppresses growth and motility of human hepatocellular carcinoma cells."
BIOMEDICINE AND PHARMACOTHERAPY (2018).
2. O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1796
3. NOHSC (1994). Triglycidylisocyanurate (TGIC). National Industrial Chemicals notification and Assessment Scheme (NICNAS). National Occupational Health and Safety Commission (Worksafe Australia).
4. M. Schwartz, S. Roayaie, M. Konstadoulakis, Strategies for the management of hepatocellular carcinoma, Nat. Clin. Pract. Oncol. 4 (2007) 424–432.
5. G. Atassi, F. Spreafico, P. Dumont, P. Nayer, J. Klastersky, Antitumoral effect in mice of a new triepoxyde derivative: 1, 3, 5-triglycidyl-s-triazinetrione (NSC296934), Eur. J. Cancer 16 (1980) 1561–1567.
6. M.M. Ames, J.S. Kovach, J. Rubin, Pharmacological characterization of teroxirone, a triepoxide antitumor agent, in rats, rabbits, and humans, Cancer Res. 44 (1984) 4151–4156.
7. Neidhart J A, D Derocher, Grever M R, et al. Phase I trial of teroxirone[J]. Cancer treatment reports, 1984, 68(9):1115-1119.
8. Ames, M. M., et al. "Pharmacologic Characterization of Teroxirone (Henkel Compound) in Animals and Humans." Cancer Chemotherapy and Selective Drug Development (1984).
9. Wang et al Reactive oxygen species-driven mitochondrial injury induces apoptosis by teroxirone in human non-small cell lung cancer cells[J]. Oncology letters, 14 (3)2017.
10. United States Patent 3910908. Triglycidyl isocyanurate preparation.
Related articles And Qustion
Lastest Price from 1,3,5-Triglycidyl isocyanurate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 2451-62-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-04-15
- CAS:
- 2451-62-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg