Application in Meat Products of Para -hydroxybenzoic acid esters (parabens)
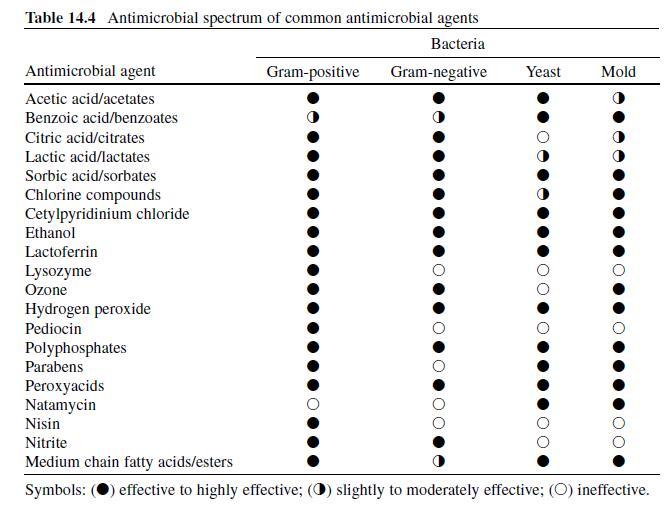
The alkyl esters (methyl, ethyl, propyl, butyl, heptyl) of para -hydroxybenzoic acid (parabens) are commonly used to preserve cosmetics and other toiletries and drugs, and, in some instances, food products (Davidson, 2005 ; Padilla-Zakour, 1998) . With the exception of methyl esters, parabens are odorless, and all impart a distinct flavor even when used at low concentrations (Davidson, 2005 ; Lueck, 1980) . As antimicrobials, parabens disorder key membrane lipids and cease membrane functions including the uptake of metabolically required nutrients and/or efflux of metabolic by-products (Bredin, Davin-Regli, & Pages, 2005 ; Tatsaguchi, Kuwamoto, Ogomori, Ide, & Watanabe, 1991) . Fungi are more susceptible to parabens than bacteria, and gram-positive bacteria are more susceptible than gram-negative types (Table 14.4 ) (Stratford & Eklund, 2003) . Alterations in cell membrane composition and subsequent resistance may be consequences of applying parabens at sublethal levels (Juneja & Davidson, 1993 ; Russell & Chopra, 1996) . Parabens with longer alkyl chains are increasingly superior as antimicrobials. Thus, the order of effectiveness is heptyl > butyl > propyl > ethyl > methyl (Aalto, Firman, & Rigler, 1953 ; Stratford & Eklund, 2003) .
 Potassium salts of parabens are highly soluble, and
therefore, more effective as antimicrobials than other less soluble paraben salts
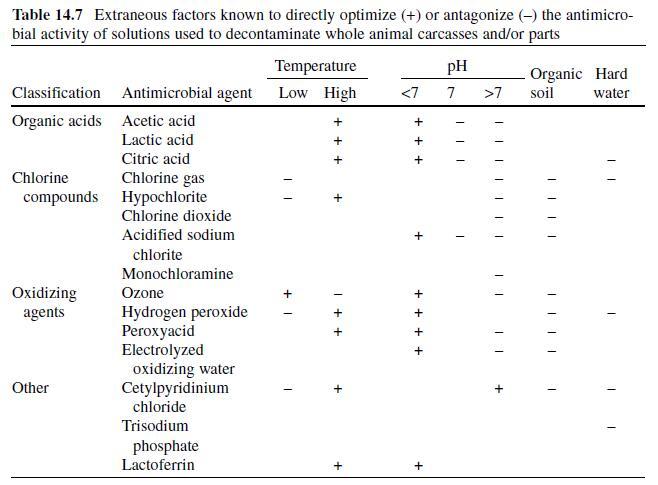
(Mizuba & Sheikh, 1987) . Activity depends on pH, temperature, time, and substrate
(Aalto et al., 1953 ; Chichester & Tanner, 1972 ; Davidson, 2005) . Parabens
are heat tolerant and increasingly soluble at elevated temperatures, yet remain
active at low temperatures (Gould, 2000 ; Padilla-Zakour, 1998 ; Smith, 2003) .
While parabens are effective over a wide pH range (3.0–8.0), antimicrobial activity
is generally optimized at a neutral to slightly alkaline pH; parabens also tend to
inhibit a larger spectrum of microorganisms when used in combination (e.g., 2:1
methyl and propyl paraben) (Gould, 2000 ; Padilla-Zakour, 1998 ; Smith, 2003) . In
contrast to other parabens which are most active at a neutral pH, potassium butyl
paraben exhibits optimal activity at pH values between 4 and 6 (Mizuba & Sheikh,
1987) . Moir and Eyles (1992) also reported that methyl paraben was more inhibitory
against L. monocytogenes at pH 5 than at pH 6. The consideration for the use
of parabens to preserve food products has raised health concerns, and although their
involvement in cases of chronic dermatitis, gastrointestinal irritation, and other forms of allergic response have been questioned, limited data are available which
link these compounds to such afflictions (Soni, Burdock, Taylor, & Greenberg,
2001 ; Soni, Taylor, Greenberg, & Burdock, 2002).
Potassium salts of parabens are highly soluble, and
therefore, more effective as antimicrobials than other less soluble paraben salts
(Mizuba & Sheikh, 1987) . Activity depends on pH, temperature, time, and substrate
(Aalto et al., 1953 ; Chichester & Tanner, 1972 ; Davidson, 2005) . Parabens
are heat tolerant and increasingly soluble at elevated temperatures, yet remain
active at low temperatures (Gould, 2000 ; Padilla-Zakour, 1998 ; Smith, 2003) .
While parabens are effective over a wide pH range (3.0–8.0), antimicrobial activity
is generally optimized at a neutral to slightly alkaline pH; parabens also tend to
inhibit a larger spectrum of microorganisms when used in combination (e.g., 2:1
methyl and propyl paraben) (Gould, 2000 ; Padilla-Zakour, 1998 ; Smith, 2003) . In
contrast to other parabens which are most active at a neutral pH, potassium butyl
paraben exhibits optimal activity at pH values between 4 and 6 (Mizuba & Sheikh,
1987) . Moir and Eyles (1992) also reported that methyl paraben was more inhibitory
against L. monocytogenes at pH 5 than at pH 6. The consideration for the use
of parabens to preserve food products has raised health concerns, and although their
involvement in cases of chronic dermatitis, gastrointestinal irritation, and other forms of allergic response have been questioned, limited data are available which
link these compounds to such afflictions (Soni, Burdock, Taylor, & Greenberg,
2001 ; Soni, Taylor, Greenberg, & Burdock, 2002).
Applications
The inhibitory effects of ethyl, propyl, and butyl parabens (0.1%) against C. botulinum toxin production in canned pork slurry were evaluated and while propyl and butyl paraben were ineffective, ethyl paraben inhibited toxin production for up to 4 weeks at 27°C (Draughon, Sung, Mount, & Davidson, 1982) . Parabens may be more effective against low levels of microbial contamination and become increasingly less effective in the presence of higher contamination levels (Padilla-Zakour, 1998) . While the use of parabens in meat products is not approved in the United States, such use is practiced in other regions of the world.
You may like
Related articles And Qustion
Lastest Price from 4-Hydroxybenzoic acid manufacturers

US $0.00/G/KG2025-04-22
- CAS:
- 99-96-7
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 1000000000KG

US $10.00/kg2025-04-21
- CAS:
- 99-96-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt