Application in Meat Products & Food Industry of Organic Acids
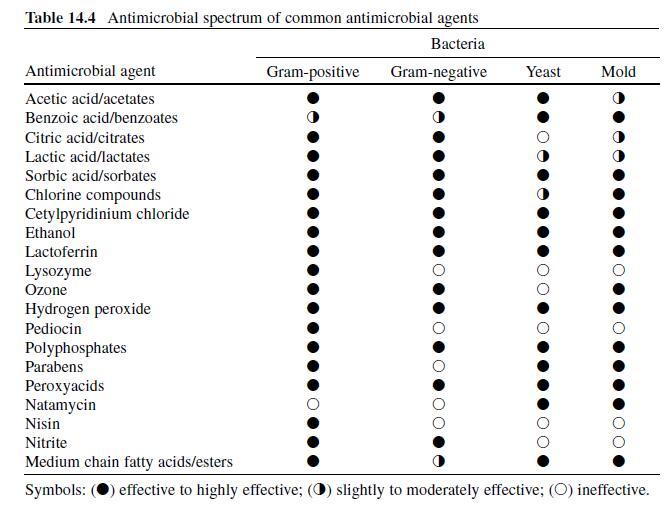
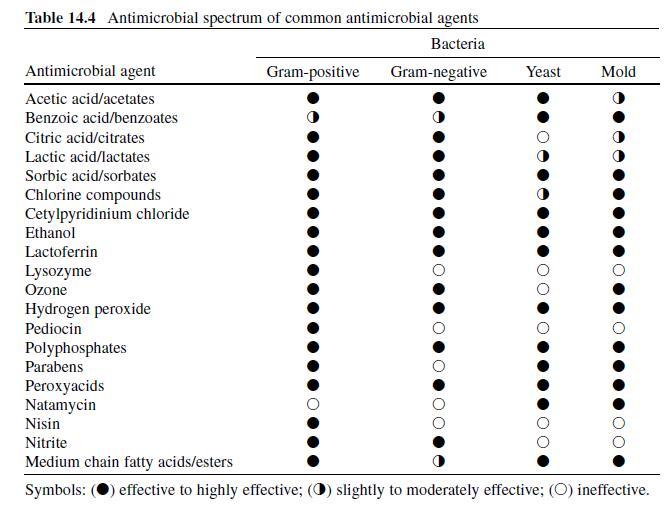
Benzoic Acid. Sodium benzoate, a derivative of benzoic acid, is a chemical preservative frequently added to food, pharmaceutical and cosmetic product formulations, although the use of sodium benzoate in food products has been scrutinized as some individuals have experienced skin rashes or asthma attacks after consuming associated food products (Sax & Lewis, 1989) . Fungi are most susceptible to benzoic acid and benzoates, although some bacteria, including pathogens and spore-formers, are also susceptible (Table 14.4 ) (Lueck, 1980 ; Sofos, 1994) . Some spoilage bacteria are impervious to benzoate compounds (Smith, 2003) . Synergistic antimicrobial activity between benzoates and other preservatives/ ingredients is also pH-dependent, and has been observed in the presence boric acid, carbon dioxide, chitosan, formic acid, parabens, sulfur dioxide, sorbic acid/ sorbates, sodium chloride, and sucrose. Benzoates are also increasingly effective in thermally processed or refrigerated food products (Lueck, 1980 ; Sagoo, Board, & Roller, 2002 ; Smith, 2003) . Lipids, ferric salts, and exposure to anionic surfactants tend to antagonize antimicrobial activity (Smith, 2003) . Topical exposures to benzoic acid may result in eye and/or dermal irritation (Sax & Lewis, 1989) .
Benzoates may possess mutagenic and teratogenic properties, and when introduced orally, may induce allergic reactions in humans (Schaubschlager, Becker, Schade, Zabel, & Schlaak, 1991) . While benzoic acid does not accumulate in the body, the acceptable daily intake of benzoic acid and its salts is limited to 0–5 mg/kg of body weight (FAO/WHO, 1966).
Sorbic Acid. The compound (C5H7-COOH) was originally isolated from the oil of unripened rowanberries (Sofos, 1989) but now it is manufactured synthetically. Sorbates, including the free acid and salt forms, are commonly used to preserve cosmetics, pharmaceuticals, and food products (Stopforth, Sofos, & Busta, 2005) and are effective against many gram-positive and -negative bacteria and fungi (Table 14.4 ) (Sofos, 2000) . Resistance to sorbate inhibition has been observed in catalase-negative LAB, yeasts, and molds including Candida , Saccharomyces , Torulopsis , Zygosaccharomyces bailii , Aspergillus , and also in some species of Pseudomonas (Smith, 2003 ; Sofos & Busta, 1993; Warth, 1988) . In one study, the addition of sorbate to a broth system did not affect growth, but did interfere with the ability of L. monocytogenes to secrete the toxin listeriolysin O (McKellar, 1993) . Of the sorbate salts, potassium sorbate is considerably more popular than calcium or sodium counterparts as it is significantly more water-soluble. Potassium sorbate is nearly 400 times more water-soluble than sorbic acid, although sorbic acid exhibits better solubility in other solvents (Sofos, 1989) . While optimal activity is observed at a pH of 4.8 or less, sorbates show measurable activity at higher (up to neutral) pH values (Smith, 2003). Antimicrobial activity is also enhanced in the presence of sulfur dioxide, carbon dioxide, sodium chloride, sucrose, propionic acid, nisin plus polyphosphates, benzoates, and in low a w foods; activity may be impaired following exposure to nonionic surfactants (Smith, 2003) . Regarding human health, sorbates are among the safest compounds used to preserve food products (Stopforth, Sofos et al., 2005). Sorbates do not appear to be mutagenic, are nontoxic at concentrations found in food products, and nonirritating for most consumers at levels used to preserve cosmetics and pharmaceuticals (Lück, 1976 ; Stopforth, Sofos et al., 2005). Sorbate-induced skin irritation has been observed in sensitive persons, with concentrations of 1% or less being sufficient to induce adverse, although minor reactions (Lück, 1976) . The acceptable daily intake of sorbic acid and its salts is limited to 0–25 mg/kg of body weight (FAO/WHO, 1974).
Other Organic Acids . Fumaric acid (HOOC-[CH] 2 -COOH) is a short-chain fatty (carboxylic) acid, which may be used to stabilize cured meat color, is effective against bacteria and molds, and has been more effective as a preservative when used in combination with sorbates (Beuchat, 1998 ; Doores, 2003 ; Podolak, Zayas, Kastner, & Fung, 1996) . Acceptable daily intake of fumaric acid is limited to 0–6 mg/kg body weight (FAO/WHO, 1974). Malic acid (HO-CH-COOH-CH 2 - COOH), the primary acid recovered from apples, cherries, and plums, is effective against bacteria and fungi and is most commonly used as a flavoring or pH control agent or to enhance the ability of antioxidants to stabilize animal fats (Doores, 2003, 2005 ; Stratford & Eklund, 2003) . Propionic acid (CH 3 -CH 2 -COOH) is effective against certain bacteria and fungi, and may be used as an antimycotic agent in food products, including processed meats (Doores, 2003, 2005) . The daily intake of malic and propionic acid is not limited (FAO/WHO, 1966). All previously mentioned compounds have received GRAS approval.
Applications
Fresh Meat Decontamination. Organic acid solutions are used to reduce microbial contamination on meat and poultry carcasses (Table 14.5), and the application of such treatments is widely practiced in the United States (Cutter & Rivera- Betancourt, 2000 ; Sofos, 2005a ; Sofos, Belk et al., 1999; Sofos, Kochevar, Bellinger et al., 1999; Sofos, Kochevar, Reagan et al., 1999; Sofos et al., 2006) . Solutions (2–5%) of organic acids (acetic, citric and lactic acid) may be applied at temperatures of up to 55°C as water-based sprays/rinses or dips ( Table 14.5 ). Lactic and acetic acid are the most commonly used organic acids in the decontamination of animal carcasses and their efficacy (2–5%), alone and in combination with other interventions, has been scientifically validated (Dickson & Siragusa, 1994 ; Hardin et al., 1995 ; Prasai et al., 1991, 1997 ; Sofos, 2005a ; Stopforth & Sofos, 2006). For instance, Cutter and Rivera-Betancourt (2000) estimated that lactic or acetic acid (2%) reduced levels of E. coli O157:H7 and Salmonella Typhimurium DT104 populations by ≥2 log CFU/cm2.
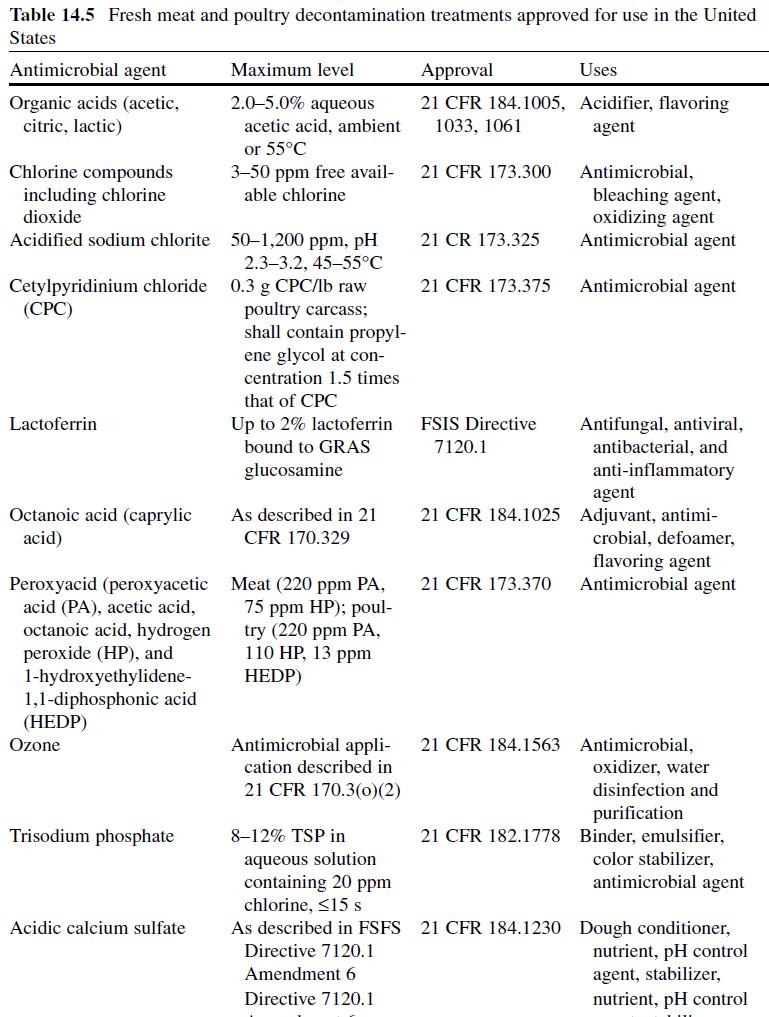
The antimicrobial activity of organic acid decontamination treatments depends on temperature, pressure, and duration of application, acid concentration, type of tissue being treated, and product storage conditions prior to and following treatment (Cords, Burnett, Hilgren, Finley, & Magnuson, 2005 ; Durán & Sofos, 2006 ; Hardin et al., 1995 ; Sofos et al., 2006) . In general, greater microbial reductions are observed when organic acids are applied at elevated temperatures (55°C) (Hardin et al., 1995) . In one study, postevisceration lactic acid spraying treatments (1.0%, 55°C) were more effective than pre-evisceration treatments in reducing microbial contamination on inoculated beef tissue (Prasai et al., 1991) . Correspondingly, lactic acid treatments (1.5%, spray) were more effective in reducing Salmonella , Listeria , and total aerobic counts on beef strip loins when applied after storage, compared to prestorage treatments (Prasai et al., 1997) . Deumier (2006) investigated the effect of commercial-scale vacuum-tumbling (1 min) deboned chicken legs in a 1% lactic acid solution on post-tumbling prevalence of Salmonella.
Citric acid and citric acid blends containing hydrochloric acid, and/or phosphoric acid, are also approved for use as poultry decontamination solutions, and may also be used to lower the pH of meat and poultry products ( Tables 14.5 and 14.6 ), although conflicting reports have been published relative to the antimicrobial effects of citric acid (Doores, 1993) . As indicated, citric acid has a much higher molecular weight than acetic or lactic acid (Table 14.3 ) and variations in efficacy may be related to the method used to dilute concentrated citric acid into working solutions. Growth of E. coli O157:H7 in fresh ground beef was inhibited following addition of sodium diacetate (0.1% or 0.2%) or sodium lactate (0.9% or 1.8%), but did not respond to buffered sodium citrate (BSC, 1% or 2%) treatments (Ajjarapu & Shelef, 1999) . Ceylan, Hajmeer, and Marsden (2002) found that 1% BSC alone did not inhibit growth of aerobic microorganisms in fresh ground beef, although 1% BSC plus 0.1% sodium diacetate maintained subspoilage levels of aerobic populations throughout storage (10 days, 4°C); the use of sodium diacetate alone was not included as a control.
Processed Meats. Sodium and calcium acetates have GRAS status and are approved for miscellaneous and general use, and also for use (≤0.25%) as antimicrobial agents in meat and poultry products (Table 14.6). Sodium and potassium lactate, individually or in combination with diacetate, may be used as antimicrobial agents in fully cooked meat, meat products, and poultry and poultry products, except those included in infant foods/formulas (Table 14.6). Citric acid may be applied to bologna in edible and inedible casings and to other meat and poultry products in fibrous casings, just before the product is sliced.