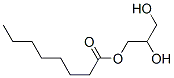
Monocaprylin: an alternative food preservative
Monocaprylin, a monoglyceride of caprylic acid, has a similar safety status and is used as an alternative food preservative. It was reported that caprylic acid and monocaprylin inhibited a variety of foodborne pathogenic and spoilage microorganisms, such as Streptococcus spp., E. coli O157:H7, Listeria monocytogenes, Enterobacter sakazakii, saprophytic molds, and avian influenza virus.

Synthesis of monocaprylin
1 eq of a short chain fatty acid (C8-C18), 20 eq of glycerol were dissolved in t-butanol. Posteriorly, 5% weight of Novozym 435 were added and the reaction ran for 2 hours at room temperature. Subsequently, the enzyme was removed by filtration, the mixture was evaporated down and the reaction mixture was dissolved in DCM. Following, the DCM layer was washed with a saturatedaqueous solution of sodium carbonate to remove the fatty acid that did not react. Later, the organic layer was washed with a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness. Finally, 2 eq of the resulted monoglyceride, 1 eq. behenic acid, 1 eq. of EDC (1-Ethyl-3-(3-dimethylaminopropyl)- carbodiimide), 0.4 eq. of DMAP (4-Dimethylaminopyridine) were made react in DCM at40°C for 45mins. After 45 minutes had elapsed, the reaction mixture was diluted in DCM and washed off with a saturated aqueous solution of ammonium chloride, a saturated solution of sodium carbonate, and brine solution. Later, the organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness and the resulting solid was dissolved in hexane and the unreacted monoglyceride was washed off with 80% Ethanol in water. If necessary, column chromatography was carried out using 8:2 Hexane: Ethyl acetate as eluent.[1]
Antimicrobial Mechanism of Monocaprylin
In contrast to diglycerides and triglycerides, monoglycerides usually have higher antimicrobial activity than their fatty acid counterpart, and this activity is strongly affected by the type of head group. Otherwise, monoglyceride's activity depends on many of the same factors as seen for fatty acids, e.g., the length of the acyl chain and the presence, number, position, and orientation of double bonds in the chain. Monocaprylin, the monoglyceride of caprylic acid (C8:0), is generally regarded as safe in the United States. Caprylic acid is present in coconut and babassu oil and milk fat. Monocaprylin is active against major food-borne pathogens like Escherichia coli O157:H7, Listeria monocytogenes, Streptococcus spp., and Yersinia spp., food-spoilage fungi such as Penicillium spp. and Aspergillus spp., and herpes simplex virus. The mode of action for monocaprylin has not yet been investigated, and we hypothesize that it is similar to that of other monoglycerides. The amphipathic monoglycerides form micelles that penetrate the cell membrane and alter membrane permeability. Electron microscopy studies showed that monolaurin (C12:0) lysed L. monocytogenes cells and monocaprin (C10:0) disrupted the cell membrane of Chlamydia trachomatis and a group B streptococcus.[2]
Scientists observed one noticeable discrepancy between experiments with microbial cells and with model membrane systems: the membrane vesicles made from E. coli lipids were permeabilized at concentrations much below the MIC of E. coli cells. Two important differences between the two experiments are the strong curvature and the absence of proteins in the 100-nm vesicles. Knowing that monocaprylin integrates into the lipid bilayer, our results indicate the membrane curvature also affects its susceptibility toward monocaprylin. Monocaprylin is, like all alkyl chains, a sensor of membrane curvature. Highly curved membranes have more defects that expose the hydrophobic bilayer core and therefore will recruit larger densities of amphiphilic molecules. The reason we did not see higher calcein release from vesicles could be because monocaprylin either caused transient disruption of the calcein-loaded vesicles or induced calcein release in a concentration-independent manner. The latter explanation suggests that monocaprylin affects membranes in an “all or nothing” mechanism, where some vesicles are permeabilized while others are not. However, a conclusive statement about the cause of the low calcein release from vesicles cannot be made without further investigations.
In summary, monocaprylin's mode of action somewhat resembles that of free fatty acids or surfactants, which form transient or permanent pores in the membrane or even solubilize membranes. The observation that monocaprylin only integrates into the Ld phase of the membrane indicates that membrane fluidity and phospholipid composition are key factors in determining an organism's susceptibility. The significance of these parameters might explain the large variation in susceptibility to monocaprylin among different species of bacteria, yeast, and fungi. Furthermore, environmental parameters that influence membrane fluidity should be considered if using monocaprylin as a food preservative.
Monocaprylin against Escherichia coli and Staphylococcus aureus
Monocaprylin showed higher bactericidal potency than caprylic acid in experiments with bacteria, such as Dermatophilus congolensis, E. coli O157:H7, and Salmonella spp. . In addition, previous studies found that the caprylic acid and monocaprylin inactivated bacteria by targeting various essential processes that occurred within and on the bacterial membrane. The reported activities of caprylic acid include inhibition of E. coli O157:H7 by disruption of the bacterial membrane, plasma membrane disintegration of D. congolensis, and damage of cytoplasmic structures and cell aggregation of Cronobacter strains. Although a plethora of information is available on the antibacterial activities of monocaprylin, its antibacterial stability toward pH and temperature and underlying mechanism against pathogens has not yet been thoroughly understood. Results showed that monocaprylin exhibited an excellent antibacterial activity against both strains, with the lowest MIC and MBC of 1.28 mg/mL. A MIC of monocaprylin remained unchanged despite the pH values of culture medium, ranging from 5 to 9, unlike that of potassium sorbate or sodium benzoate. Furthermore, monocaprylin at MBC effectively reduced the population of E. coli and S. aureus by >5.5 log CFU/mL at 25°C within 6 h and decreased E. coli by approximately 5.0 log CFU/mL and S. aureus by 2.9 log CFU/mL at 12 h. [3]
In summary, monocaprylin exhibited stronger and more stable antibacterial activities against foodborne bacteria (E. coli and S. aureus) than sodium benzoate and potassium sorbate in vitro. The mode of action against E. coli is a rapid change in permeability and integrity of the disrupted membrane, resulting in the decline of membrane potential, leakage of nucleic acids and proteins, and ultimately cell membrane disintegration and lysis. Monocaprylin may diffuse across the cell wall of S. aureus, decrease membrane potential, disturb intracellular contents, and collapse the cell membrane. Given the safety and reliability of monocaprylin over conventional preservatives, monocaprylin merits consideration as a novel food preservative.
References
[1] KLINGE PHARMA - WO2017/74902, 2017, A1
[2] Hyldgaard M, Sutherland DS, Sundh M, Mygind T, Meyer RL. Antimicrobial mechanism of monocaprylate. Appl Environ Microbiol. 2012 Apr;78(8):2957-65.
[3] Wang J, Ma M, Yang J, Chen L, Yu P, Wang J, Gong D, Deng S, Wen X, Zeng Z. In Vitro Antibacterial Activity and Mechanism of Monocaprylin against Escherichia coli and Staphylococcus aureus. J Food Prot. 2018 Dec;81(12):1988-1996.
You may like
See also
Lastest Price from MONOCAPRYLIN manufacturers

US $10.00/KG2025-04-21
- CAS:
- 26402-26-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $6.00/kg2025-04-21
- CAS:
- 26402-26-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month