Determination methods of the Sodium Methylparaben
Introduction
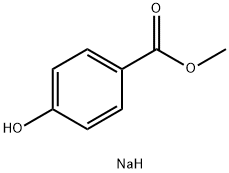
As a type of preservative, sodium methylparaben (Figure 1) can damage bacterial cell membranes and inhibit bacterial growth. It has good antibacterial properties in both acidic and alkaline environments. Sodium methylparaben is a salt that is easily soluble in water, has good dispersibility, and has better preservative ability than p-hydroxybenzoate. Therefore, it is widely used in the preservation and preservation of beverages, dairy products, and pastries.

1.Determination of the Sodium Methylparaben Content Based on Spectrum Fluorescence Spectral Technology and GA-BP Neural Network
Sodium methylparaben as one kind of preservatives is widely used in our life, but it will do harm to health if it is eaten too much. So there are strict rules on the dosage of sodium methylparaben in every country. The fluorescence spectral properties of sodium methylparaben in aqueous solution and orange juice solution are analyzed with FS920 fluorescence spectrometer. The research result shows that the fluorescence characteristic peak of sodium methylparaben solution is in λ(ex)/λ(em) = 380/5 10 nm, while sodium methylparaben orange juice solution has two fluorescence characteristic peaks which are in λ(ex)/λ(em) = 440/520 nm and 470/530 nm, and its best excitation wavelength is 440 nm. So it can be concluded from the result that there is a significant change between the characteristic peaks of sodium methylparaben in the two solution. Compared with the fluorescence characteristic peak of sodium methylparaben solution, thoses of sodium methylparaben orange juice solution are changed significantly, which are caused by the interference of orange juice fluorescence characteristics. In order to determine the content of sodium methylparaben in the fresh orange juice, a detection model of sodium methylparaben content in orange juice is built based on GA-BP neural network, according to the relationship between fluorescence intensity in λ(ex) = 440 nm and the content of sodium methylparaben orange juice solution. When the accuracy of the mean square error in the process of network training reaches 10(-3), the correlation coefficient of network output and that of the expected is 0.996. At the same time, a better prediction result can be obtained that the average recovery of the forecast samples is 98.67% and the average relative standard deviation is 0.86%. When the concentration ranges from 0.02 to 1.0 g x L(-1), the results testify that detection method based on fluorescence spectroscopy and GA-BP neural network can accurately determine the content of sodium methylparaben in orange juice. This method has the features of novelty and simplicity and it is expected to be applied to the determination of sodium methylparaben in other kinds of drink.[1]
2. Validated Stability-indicating Reverse-phase Ultra-performance Liquid Chromatography Method for Determination of Sodium Methylparaben
A detailed literature survey revealed that a number of methods are available for determination of KTR, sodiummethylparaben (SMP) and sodium propylparaben (SPP) individual in serum by high-performanceliquid chromatography (HPLC), gas chromatographymass spectrometry, spectrophotometry, liquidchromatography mass spectrometry, and inpharmaceutical dosage form by HPLC
A sensitive, fast, and stability-indicating isocratic reverse-phase ultra-performance liquid chromatography method was developed and validated for quantitative simultaneous determination of sodium methylparaben, sodium propylparaben and ketorolac tromethamine in topical dosage forms. Separation of all peaks was achieved by using acquity ethylene bridged hybrid C18 (50×2.1 mm, 1.7 μ) as stationary phase, mobile phase used was triethylamine buffer (pH 2.5):tetrahydrofuran:methanol (665:35:300, v/v/v) with isocratic mode at a flow rate of 0.40 ml/min. All component were detected at 252 nm with 10 min run time. The described method was found to be linear in the concentration range of 248-744 μg/ml for ketorolac tromethamine, 20.8-62.4 μg/ml for sodium methylparaben and 2.38-7.13 μg/ml for sodium propylparaben with correlation coefficients more than 0.999. Method was validated in terms of specificity, linearity, accuracy, precision, solution stability, filter equivalency, and robustness as per International Conference on Harmonization guideline. Formulation was exposed to the stress conditions of peroxide, acid, base, thermal, and photolytic degradation and proven all components were well separated in the presence of degradants. The developed liquid chromatography method was validated with respect to specificity,linearity, precision, accuracy, solution stability, filter equivalency, and robustness. Force degradation studies were performed on the placebo and drug product. Developed method separated all degradation products from KTR, sodiummethylparaben and sodium propylparaben and exhibits stability indicating nature.[2]
References
[1] Wang ST, Chen DY, Hou PG, Wang XL, Wang ZF, Wei M. Guang Pu Xue Yu Guang Pu Fen Xi. 2015;35(6):1606-1610.
[2] Roy C, Chakrabarty J, Modi PB. Validated Stability-indicating Reverse-phase Ultra-performance Liquid Chromatography Method for Simultaneous Determination of Sodium Methylparaben, Sodium Propylparaben and Ketorolac Tromethamine in Topical Dosage Forms. Indian J Pharm Sci. 2013;75(2):197-204.
You may like
Lastest Price from Sodium methylparaben manufacturers

US $1.00/KG2025-11-06
- CAS:
- 5026-62-0
- Min. Order:
- 1KG
- Purity:
- 99.0-102.0%
- Supply Ability:
- 20Tons/month

US $500.00/kg2025-04-21
- CAS:
- 5026-62-0
- Min. Order:
- 100kg
- Purity:
- 99.1%
- Supply Ability:
- 5 tons