Multifaceted Studies on Octadecyltrimethoxysilane: From Air/Water Interface Reactions to Nanodevice Applications
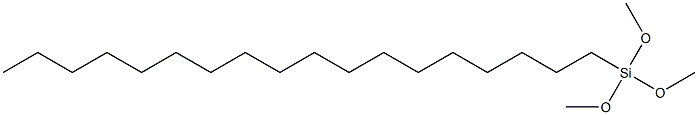
Octadecyltrimethoxysilane (OTMS) is an organosilicon compound. This colorless liquid is used for preparing hydrophobic coatings and self-assembled monolayers. It is sensitive toward water, irreversibly degrading to a siloxane polymer. It places a C18H39SiO3 "cap" on oxide surfaces. The formation of octadecyltrimethoxysilane monolayers is used for converting hydrophilic surfaces to hydrophobic surfaces, e.g. for use in certain areas of nanotechnology and analytical chemistry.

Separating Octadecyltrimethoxysilane Hydrolysis and Condensation at the Air/Water Interface
Hydrolysis and condensation of chloro- and organo-silanes at the air/water interface have been the subjects of several recent studies. The silane most commonly employed in these studies, octadecyltrimethoxysilane, OTMS (CH3–(CH2)17–Si–(OCH3)3), undergoes a 2-D sol–gel process which is strongly dependent on subphase pH, as well as monolayer surface pressure and incubation time (including monolayer spreading and solvent evaporation times). The interfacial reactivity of the OTMS sol–gel process have been monitored either as molecular area (A) or surface pressure (π) changes over time. The resultant octadecyltrimethoxysilane isobars and iso-area curves display features which have been attributed to the formation of protonated reaction intermediates (A or π increases) followed by hydrolysis and condensation of these intermediates (A or π decreases). Numerous OTMS hydrolysis and condensation paths exist as depicted schematically. In this diagram an octadecyltrimethoxysilane molecule is represented as (x,y,z) where x is the number of –OR substitutions, y the number of –OH substitutions, and z the number of –O–Si substitutions on the silicon atom. As has been previously recognized, confining the sol–gel process to the air/water interface improves the probability of condensation-producing collisions between silane monomers, and condensation is expected to occur immediately following hydrolysis.[1]
An accelerated hydrolysis and condensation for octadecyltrimethoxysilane monolayers containing SME are apparent in both the A(t) isobars and the fluorescence images presented. Both the molecular area increase features and the time required to reach a limiting molecular area decreased in proportion to the concentration of SME. A dramatic influence of SME on condensed domain morphology, size, and monodispersity was observed as well. The molecular area increase feature in the A(t) isobars, attributed to protonation of octadecyltrimethoxysilane prior to hydrolysis/condensation, is not apparent for the 1:6 OTMS:SME molar ratio. This finding is expected since OTMS is diluted in a matrix of SME; moreover, protonated OTMS species are less likely to encounter (repulse) one another. However, as the OTMS:SME ratio is increased to 7:3, a molecular area increase reappears in the isobar, suggesting that a 7:3 OTMS:SME ratio only partially screens electrostatic repulsion among octadecyltrimethoxysilane intermediates. For the 98:2 OTMS:SME ratio, electrostatic repulsion is prevalent and two molecular area increases are observed, possibly corresponding to a stepwise protonation of methoxy groups on OTMS as previously reported. Nonetheless, even 2% SME is sufficient to accelerate the reaction rate 2-fold (reaction complete in 15 h) as compared to the pure octadecyltrimethoxysilane monolayer which does not reach a limiting molecular area until 30 h.
The hydrolysis and condensation of octadecyltrimethoxysilane (OTMS) at the air/water interface were monitored through molecular area changes at a constant surface pressure of 10 mN/m. The onset of condensation was delayed through the addition of methyl stearate (SME) acting as an inert filler molecule. In the absence of SME, complete gelation of OTMS required 30 h, during which time OTMS condensation occurred concomitantly with hydrolysis. In the presence of SME, the octadecyltrimethoxysilane monolayer gelation rate increased in proportion to the amount of SME present. A 1:6 OTMS:SME molar ratio resulted in monolayer gelation within 30 min, suggesting completion of monomer hydrolysis prior to condensation. These findings indicate that lability of octadecyltrimethoxysilane to hydrolysis at the air/water interface is governed by steric and conformational constraints at the silicon atom site, with monomeric OTMS being much more reactive than oligomeric OTMS. Fluorescence microscope images demonstrated that the octadecyltrimethoxysilane condensed domain size also decreased with increasing SME concentrations, further implicating SME’s role as an inert filler.
Stability of Octadecyltrimethoxysilane-Based Coatings
Long-term stability in contact with water of organosilane layers formed by octadecyltrimethoxysilane (ODTMS) on polished aluminum alloy (AA2024) through dip-coating was studied by combining SEM, water contact angle measurements, and X-ray photoelectron spectroscopy. This work aimed at systematically studying the hydrophobic properties of polished (flat) aluminum surfaces coated with an octadecyltrimethoxysilane (ODTMS) layer as they were immersed in water. Both surfaces coated with ODTMS (as one layer) and with an under-layer and ODTMS as a top-layer were tested. We used the following coatings as under-layers: (i) permanganate conversion coating, (ii) organosilane layer based on BTSE, and (iii) hydrated SiOx layer prepared through hydrolysis of TEOS. For comparison, another one-layer ODTMS sample was subjected to the ultrasonic rinse test, which simulated a long-term water immersion test over a short period of time. All the samples showed gradual decay of their water-repellent properties, which should be related to gradual hydrolysis of octadecyltrimethoxysilane molecules grafted to the surface via their Si-O group.[2]
To study their long-term stability in contact with water, all the samples were immersed in deionized water, and their water contact angle was measured systematically for as long as several weeks (typically at least 2–4). For comparison, another one-layer octadecyltrimethoxysilane sample was subjected to the ultrasonic rinse test, which simulated a long-term water immersion test over a short period of time. The highest stability over time was demonstrated by a one-layer sample prepared in ethanol/water bath for 5 min and by a similar octadecyltrimethoxysilane layer prepared on hydrated MnOx as an under-layer. As all the samples exhibited a decrease in their hydrophobicity over time, it is concluded that ODTMS layers get hydrolyzed gradually and slowly degrade, letting water molecules interact with their underlying layers. This was also confirmed by both SEM and XPS investigations. Since octadecyltrimethoxysilane coatings prepared on Al alloy via dip-coating proved to be unstable, for comparison, their counterpart layers prepared via CVD should be tested in the future.
octadecyltrimethoxysilane monolayers for the selective deposition of nanoparticles on silicon substrate
The use of nano-objects to make the active part of reproducible nanodevices requires their controlled assembling on specific areas of substrates. In this work, scientists propose to use van der Waals interactions to assemble selectively gold particles covered by alkyl-thiol ligands on hydrophobic OctadecylTriMethoxySilane (OTMS) patterns defined on SiO2/Si substrates by a process combining nano-imprint lithography (NIL) or high resolution electron beam lithography (HREBL) and atmospheric chemical vapor deposition (CVD) of silane. Contact angle measurements of a water drop on non-patterned substrates confirm the quality of the OTMS deposition. As expected, the substrates covered by octadecyltrimethoxysilane molecules are highly hydrophobic with contact angles of 109°, comparable to those reported in the literature.[3]
First depositions of gold nanoparticles on these octadecyltrimethoxysilane patterns clearly reveal a selective assembling of the nanoparticles on the chemical patterns. In fact, as evidenced, there is a higher density of nanoparticles (bright spots) on the octadecyltrimethoxysilane patterns (dark areas) than on the bare substrate. As it was demonstrated in a previous work, this selectivity is due to the stronger affinity of ethanol with the bare SiO2 than with the OTMS patterns, whereas the nanoparticles are only linked to the surface by van der Waals non-specific interactions. A study by atomic force microscopy (AFM) reveals that homogeneous patterns of octadecyltrimethoxysilane self-assembled monolayers, extending on several square millimeters, have been made. These OTMS patterns, with a lateral dimension ranging from 2 μm down to 50 nm, can be located at a precise place of a nanodevice, for example, between nanoelectrodes. Preliminary results of selective nanoparticle deposition on these chemical patterns are presented.
References
[1]Britt DW, Hlady V. Separating Octadecyltrimethoxysilane Hydrolysis and Condensation at the Air/Water Interface through Addition of Methyl Stearate. J Phys Chem B. 1999 Apr 8;103(14):2749-2754.
[2]Zhizhchenko AY, Shabalina AV, Aljulaih AA, Gurbatov SO, Kuchmizhak AA, Iwamori S, Kulinich SA. Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface. Materials (Basel). 2022 Feb 28;15(5):1804.
[3]Ressier L, Viallet B, Grisolia J, Peyrade JP. Chemical patterns of octadecyltrimethoxysilane monolayers for the selective deposition of nanoparticles on silicon substrate. Ultramicroscopy. 2007 Oct;107(10-11):980-4.
See also
Lastest Price from Octadecyltrimethoxysilane manufacturers

US $99.00-45.00/kg2025-04-21
- CAS:
- 3069-42-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton
US $0.00-0.00/kg2025-04-18
- CAS:
- 3069-42-9
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 96%
- Supply Ability:
- 200tons