Acetic Acid - Application in Meat Processing & Food Industry
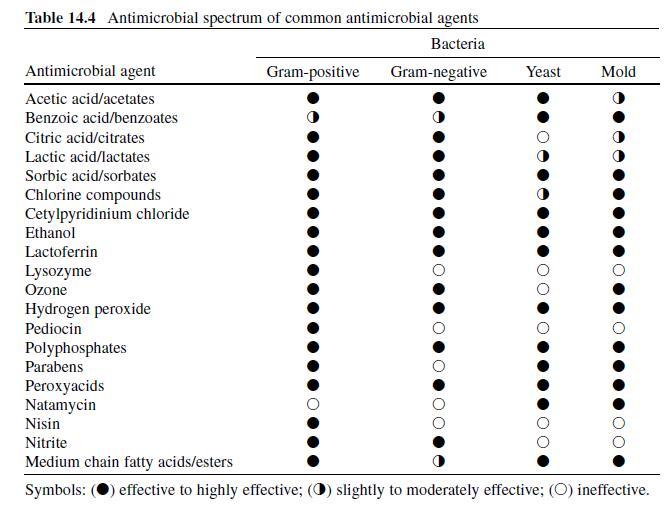
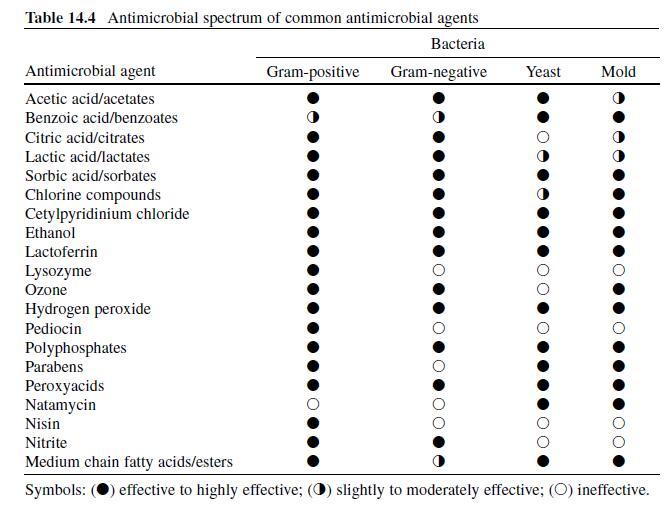
Acetic or ethanoic acid (CH 3 -COOH; log P oct – 0.319) is a monocarboxylic acid which occurs naturally in plant and animal tissues and is also a byproduct of ethanol oxidation by Acetobacter , Gluconobacter and other heterofermentative strains of lactic acid bacteria (LAB) (Doores, 2003 ; Leo et al., 1971) . Homofermentative LAB, gram-negative and gram-positive bacteria, yeasts, and some mold species exhibit various degrees of sensitivity to acetic acid (Table 14.4 ) (Doores, 2005) . Vinegar, a 5% acetic acid solution, is known for its strong sensory characteristics, and thus acetic acid is self-limiting in food applications. As an antimicrobial agent, acetic acid (2–5% solutions) is used to decontaminate food animal carcasses and potassium and sodium acetate/diacetate may also be added to processed meat and poultry products to control L. monocytogenes in combination with sodium/potassium lactate (Tables 14.5 and 14.6 ). Other organisms inhibited by sodium diacetate include pathogenic E. coli , Pseudomonas fluorescens , Salmonella , Shewanella putrefaciens , Bacillus cereus , and some aerobic microorganisms involved in spoilage (Ajjarapu & Shelef, 1999 ; Shelef & Addala, 1994) . Acetic and lactic acid as well as lactate and diacetate blends may demonstrate synergistic properties when used in combination (Adams & Hall, 1988 ; Barmpalia et al., 2005 ; Blom et al., 1997) . Daily intake of acetic acid and calcium, potassium or sodium acetate is not limited for humans of any age and acetic acid may be used in infant formulas (Food and Agriculture Organization/World Health Organization [FAO/ WHO], 1966, 1974). The acceptable daily intake of sodium diacetate for humans is 15 mg/kg of body weight (FAO/WHO, 1974).
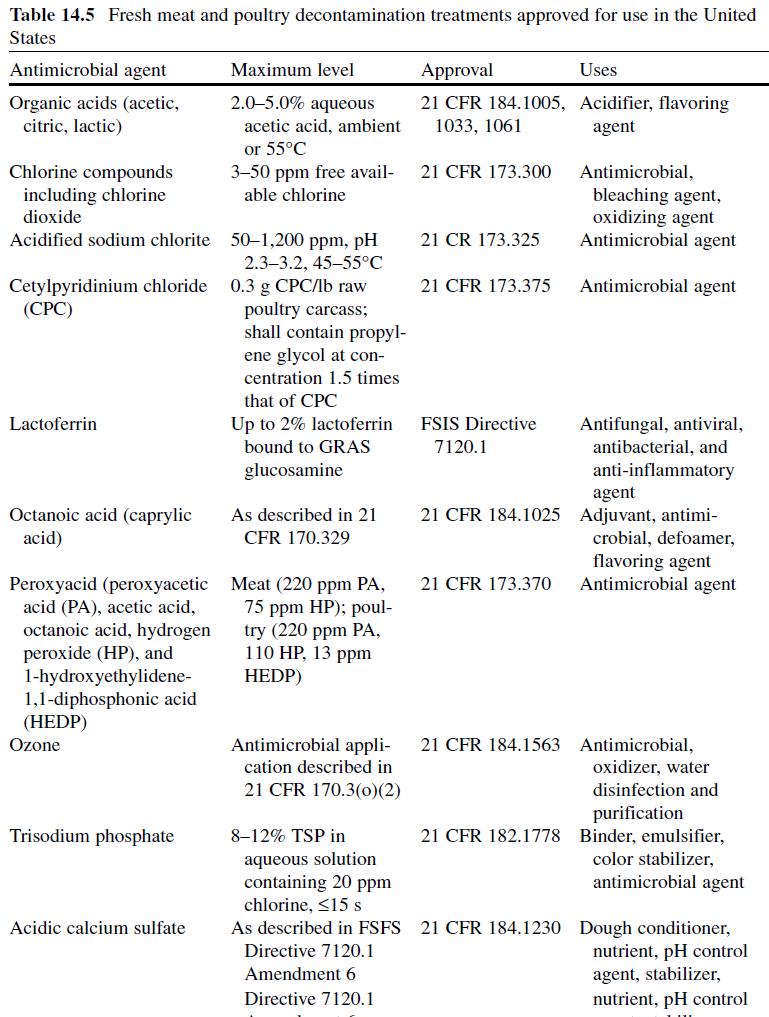
Citric Acid . Citric acid (C6H3O7 ; log Poct – 0.172) can be isolated from citrus fruits and is produced during normal metabolic processes of most living organisms (Doores, 2003 ; Leo et al., 1971) . It is a powerful chelator which sequesters metal ions (Ca 2+ , Mg 2+ , Fe 3+ ) involved in oxidative rancidity and also required for microbial homeostasis (Stratford & Eklund, 2003) . Citric acid is used throughout the food industry as a sequesterant, dispersing agent, acidifier, flavoring agent, preservative, or as a dietary supplement (manganese citrate) and may also be used in infant formulas (Doores, 2003 ; Institute of Medicine [IOM], 2003). Aqueous solutions (2–5%) may be used to decontaminate animal carcasses ( Table 14.5 ) and multiple citric acid derivatives (isopropyl and monostyryl citrate) are used to preserve the color of cured meats and/or promote antioxidant efficacy (Doores, 2003) . As applied in commercial decontamination treatments, citric acid may provide less antimicrobial activity because (1) it is nonlipophilic (Leo et al., 1971) , (2) its p K a is quite low (3.14), (3) it is produced during normal metabolic processes and may be easily metabolized by numerous microorganisms (Foegeding & Busta, 1991) , and (4) it has a higher molecular weight than other organic acids (Table 14.3 ).
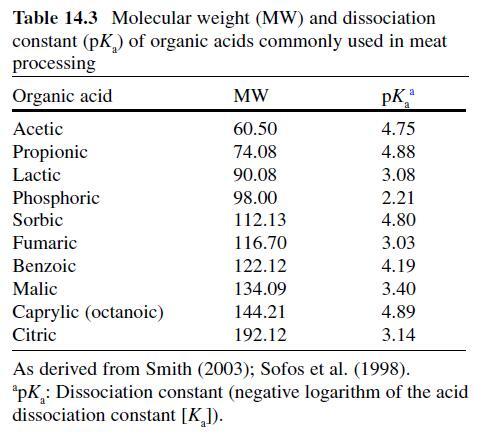
Therefore, it may be less likely to be taken up into cells or be applied at lower concentrations than other acids when using percentage-based working solutions. When compared at equal molar concentrations, citric acid was more effective as an antimicrobial than lactic or acetic acid (Durán & Sofos, 2006 ; Miller, Call, & Whiting, 1993) . Buchanan and Golden (1994) found that the antilisterial activity of citric acid solutions was reduced at pH values >5, especially when the concentration of citric acid was low (≤0.5%). The daily intake of citric acid/derivatives is not limited (FAO/WHO, 1966).
Lactic Acid. Lactic acid (CH 3 -CH(OH)-COOH; log P oct – 0.620) is produced during fermentation by LAB, and is available as a hygroscopic, syrupy liquid, with a moderately strong acidic taste (Doores, 2005 ; IOM, 2003; Leo et al., 1971) . In addition to the decontamination of beef, pork, and poultry carcass, lactic acid is a multifunctional ingredient and approved for use as an acidulant, flavoring agent, emulsifier, and antimicrobial agent in various meat and poultry product formulations ( Tables 14.5 and 14.6 ) (Shelef, 1994 ; Sofos, Belk, & Smith, 1999 ; Sofos, Beuchat, Davidson, & Johnson, 1998 ; Sofos, Kochevar, Bellinger, et al., 1999; Sofos, Kochevar, Reagan, & Smith, 1999 ; Sofos et al., 2006 ; Wederquist, Sofos, & Schmidt, 1994) . Organisms inhibited by lactic acid include gram-negative and -positive bacteria and fungi (Table 14.4 ) (Sofos, Maga, & Boyle, 1988 ). Two isomers of lactate (d- and l-lactate) exist, either of which may be produced during the normal metabolic processes of some microorganisms (FAO/WHO, 1966), and commercial solutions containing the individual isomers, or equal concentrations of d- and l-lactate, are available. In one study, the antimicrobial effect of d-lactate (≤150 mM) against E. coli O157 and non-O157 strains was inferior to equal concentrations of l-lactate; however, both isoforms were equally lethal at concentrations ≥200 mM (McWilliam Leitch & Stewart, 2002) . This phenomenon is attributed, in part, to the generation of d-lactate by E. coli during fermentation, requiring these organisms to possess cellular mechanisms to regulate its presence inside the cell (Bunch, Mat-Jan, Lee, & Clark, 1997 ; McWilliam Leitch & Stewart, 2002).
In general, the efficacy of lactic acid is enhanced in the presence of other bacteriostatic factors produced by LAB (e.g., bacteriocins), acetates and diacetates, potassium sorbate, nisin, nitrites, glucono-delta-lactone, ethanol, and under modified atmosphere conditions (Doores, 2005 ; Jordan et al., 1999 ; Malicki et al., 2004 ; Scannell, Hill, Buckley, & Arendt, 1997) . The acceptable daily intake of lactic acid is not limited, although its calcium, potassium, and sodium salts are limited to 100 mg/kg/day of dl-lactate (FAO/WHO, 1966). l- and dl-Lactic acid/lactates should not be used in infant formulas (Doores, 2003) .
You may like
Related articles And Qustion
See also
Lastest Price from Acetic acid manufacturers

US $1.90/KG2025-06-10
- CAS:
- 64-19-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton

US $1.00/KG2025-04-21
- CAS:
- 64-19-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt