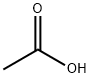
What is Acetic acid?
Acetic acid is present throughout nature as a normal metabolite of both plants and animals. Acetic acid may also be released to the environment in a variety of waste effuents, in emissions from combustion processes, and in exhaust from gasoline and diesel engines.

Preparation
Acetic acid is produced by the decomposition of solid biological wastes and is readily metabolized by living organisms. Using bacteria (Acetobacter species), large quantities of vinegar are manufactured by fermenting alcohol employing the following reaction: C2H5OH+O2→CH3COOH+H2O
Uses
Large quantities of acetic acid are used to make products such as photographic chemicals, pesticides, pharmaceuticals, food preservatives, rubber, and plastics. Acetic acid is also present as the main component of vinegar, albeit at very low concentrations that are harmless to humans. Acetic acid is used in the manufacture of various acetates, acetyl compounds, cellulose acetate, acetate rayon, plastics, and rubber. It is also used in tanning, as laundry sour, in printing calico, and in dyeing silk. It is a solvent for gums, resins, volatile oils, and many other substances. Acetic acid is widely used in commercial organic synthesis.
Mechanism of Toxicity
Acetic acid is present throughout nature as a normal metabolite of both plants and animals. Acetic acid may also be released to the environment in a variety of waste effuents, in emissions from combustion processes, and in exhaust from gasoline and diesel engines. If released to air, a vapor pressure of 15.7 mmHg at 25 °C indicates acetic acid should exist solely as a vapor in the ambient atmosphere. Vapor-phase acetic acid will be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 22 days.
Physical removal of vapor-phase acetic acid from the atmosphere occurs via wet deposition processes based on the miscibility of this compound in water. In acetate form, acetic acid has also been detected in atmospheric particulate material. If released to soil, acetic acid is expected to have very high to moderate mobility based upon measured Koc values, using near-shore marine sediments, ranging from 6.5 to 228. No detectable sorption was measured for acetic acid using two different soil samples and one lake sediment.
Volatilization from moist soil surfaces is not expected to be an important fate process based upon a measured Henry’s law constant of 1×10-9 atmm3 mol-1. Volatilization from dry soil surfaces may occur based upon the vapor pressure of this compound. Biodegradation in both soil and water is expected to be rapid; a large number of biological screening studies has determined that acetic acid biodegrades readily under both aerobic and anaerobic conditions. Volatilization from water surfaces is not expected to be an important fate process based on its measured Henry’s law constant. An estimated bacterial colony foraging (BCF) of <1 suggests that the potential for bioconcentration in aquatic organisms is low.
Environmental Fate
Acetic acid causes toxicity by coagulative necrosis; that is, the acid denatures all tissue proteins to form an acid proteinate. As a result, both structural and enzymatic proteins are denatured and cell lysis is blocked. Therefore, cell morphology is not greatly interrupted. In addition, an ester is formed which delays further corrosive damage and helps reduce systemic absorption. Thus, damage, especially with small quantities of acid, is frequently limited to local sites of injury to the skin or the GI tract rather than the systemic response.
Related articles And Qustion
Lastest Price from Acetic acid manufacturers

US $5.00/Box2026-01-08
- CAS:
- 64-19-7
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 20000 boxes

US $1.90/KG2025-06-10
- CAS:
- 64-19-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton