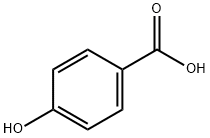
4-Hydroxybenzoic acid: Synthesis method and Biological activity
Introduction
4-hydroxybenzoic acid (4-HBA) is a monohydroxybenzoic acid that is benzoic acid carrying a hydroxy substituent at C-4 of the benzene ring. It has a role as a plant metabolite and an algal metabolite. It is a conjugate acid of a 4-hydroxybenzoate.
It can be isolated naturally from carrots(Daucus carota), oil palm (Elaeis guineensis), grapes (Vitis vinifera), east African satinwood (Fagara macrophylla), yellow leaf tree (Xanthophyllum rubescens), peroba(Paratecoma peroba), taheebo (Tabebuia impetiginosa), red sandalwood (Pterocarpus santalinus), southern catalpa (Catalpa bognoniooides), Chinese chest tree (Vitexnegundo), betel palm (Areca catechu), Cuban royal palm(Roystonea regia) and medlar (Mespilus germanica) or can be synthesized chemically. It is also detected in cell wall extracts of Arabidopsis thaliana roots, and its concentration increased upon infection with Pythiumsylvaticum. It is also synthesized de novo in stems and petioles in response to a mobile signal by Pseudomonassyringae pv. Syringae.
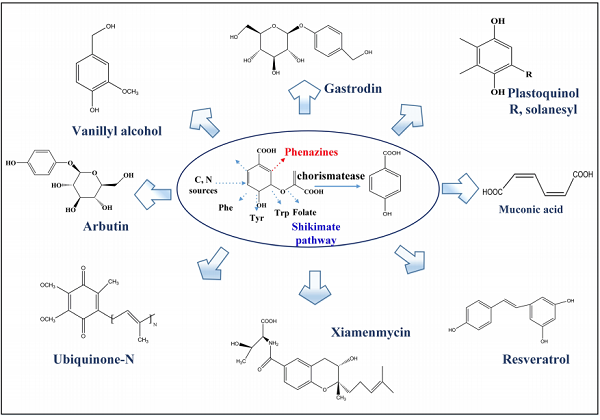
In prokaryotes, 4-HBA forms from chorismate via the shikimate pathway. As a useful industrial platform chemical, the high titer of 4-HBA can be converted into more valuable compounds like resveratrol, muconic acid, gastrodin, xiamenmycin, ubiquinone, vanillyl alcohol, and many others. The 4-HBA-derived natural products are a large group of secondary metabolites that exhibit various biological and pharmaceutical activities[1].
Synthesis method
In 1947, H. Gilman and C. E. Arntzen at Iowa State College (Ames) reported the synthesis of 4-HBA from 4-hydroxybenzaldehyde. Several other preparation methods have been developed since then. Today, it is produced commercially via the Kolbe–Schmitt reaction, which originated in the mid-1800s. Potassium phenoxide and CO2 are heated under pressure; the reaction mixture is then acidified to obtain the product. (Oddly, if the sodium salt is used, the product is 2-hydroxybenzoic acid or salicylic acid.)
Biological activity
4-hydroxybenzoic acid is reportedly antibacterial(against Gram +ve and Gram –ve bacteria), antifungal, antialgal, antimutagenic, antisickling and estrogenic activity. It is also used as a trapping agent to study hydroxyl radical generation during cerebral ischemia and reperfusion and is also widely used as a preservative in drugs, cosmetics, pharmaceuticals, food, and beverages. It showed inhibitory effects on hepatic enzyme pyrophosphate decarboxylase and mevalonate phosphate kinase while competing with substratemevalonate 5-pyrophosphate and also induces oxidative stress in the skin after conversion to glutathione conjugates of hydroquinone by reacting with singlet oxygen and glutathione. Induction and control of p-hydroxybenzoic acid under stress conditions are essential for the antioxidative system because biosynthesis of salicylic acid is catalyzed by benzoic acid 2-hydroxylase and connected with p-hydroxybenzoic acid.
Sannino et al. found that 4-hydroxybenzoic acid (4-HBA) can induce pyroptosis-regulated cell death in LC A549 cell lines. Moreover, 4-HBA can upregulate caspase-1, IL-1β, and IL-18 in LAD cells. The 4-HBA treatment can also activate the transcription of caspase-1, IL-1, and IL-18 regulatory genes in A549 cells. However, additional research is required to assess how 4-HBA induces pyroptosis-regulated death in cancer cells[2].
References:
[1] SONGWEI WANG. 4-Hydroxybenzoic acid—a versatile platform intermediate for value-added compounds[J]. Applied Microbiology and Biotechnology, 2018, 102 8. DOI:10.1007/s00253-018-8815-x.[2] CHEN HUANG C Z Jian Li. What role does pyroptosis play in cancer?[J]. Molecular Metabolism, 2022, 65. DOI:10.1016/j.molmet.2022.101587.
You may like
Related articles And Qustion
See also
Lastest Price from 4-Hydroxybenzoic acid manufacturers

US $0.00/G/KG2025-04-22
- CAS:
- 99-96-7
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 1000000000KG

US $10.00/kg2025-04-21
- CAS:
- 99-96-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt