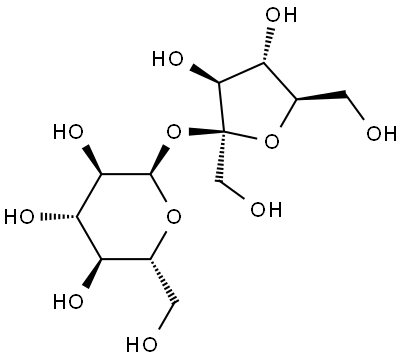
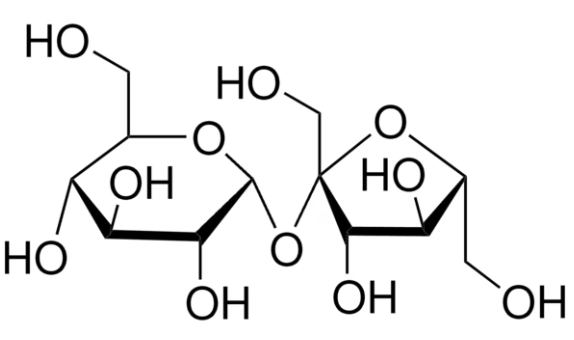
Which's the reactions of Sucrose(C12H22O11)?
Sucrose (α-d-glucopyranosyl-β-d-fructofuranoside) is a disaccharide with the general molecular formula C12H22O11. In solution, it is present primarily in the cyclic form. All sugars are fairly reactive but, in sucrose, the reactive sites of glucose and fructose are bonded together.
Chemical properties
Sucrose is a nonreducing sugar and is stable when heated. Fructose will decompose at temperatures above 103 °C, and both glucose and fructose are unstable in alkaline solutions. In acidic solutions, sucrose will invert or break down to its component monosaccharides. This reaction is accelerated with increasing acidity or temperature. In solution (including in human metabolism), most sucrose reactions begin with the inversion reaction.
Chemical reactions
Sucrose can undergo a combustion reaction to yield carbon dioxide and water.
When reacted with chloric acid, this compound yields hydrochloric acid, carbon dioxide, and water.
Upon hydrolysis, the glycosidic bond linking the two carbohydrates in a C12H22O11 molecule is broken, yielding glucose and fructose.
Sucrose can be dehydrated with the help of H2SO4 (which acts as a catalyst) to give rise to a black solid which is rich in carbon.
C12H22O11 → 12C+ 11H2O It is a decomposition reaction.
Basically, a decomposition reaction is when one big molecule is decomposed into two or more small molecules.
Hence, the reaction will be - XY equals to X + Y.
It is breakdown or separation of the molecules.
In this case, the carbon abd water molecules are being separated or we can say they are being decomposed into two parts.
You may like
Related articles And Qustion
See also
Lastest Price from Sucrose manufacturers

US $0.00/KG2025-04-21
- CAS:
- 57-50-1
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/kg2025-04-15
- CAS:
- 57-50-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000