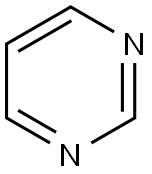
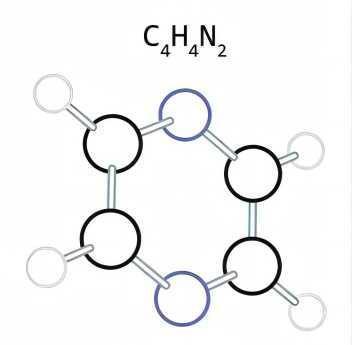
Synthesis and uses of Pyrimidine
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine (C5H5N). The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug zidovudine.
Synthesis
Pyrimidine biosynthesis creates derivatives —like orotate, thymine, cytosine, and uracil— de novo from carbamoyl phosphate and aspartate.
Pyrimidines can be prepared via the Biginelli reaction and other multicomponent reactions. Many other methods rely on condensation of carbonyls with diamines for instance the synthesis of 2-thio-6-methyluracil from thiourea and ethyl acetoacetate or the synthesis of 4-methylpyrimidine with 4,4-dimethoxy-2-butanone and formamide.
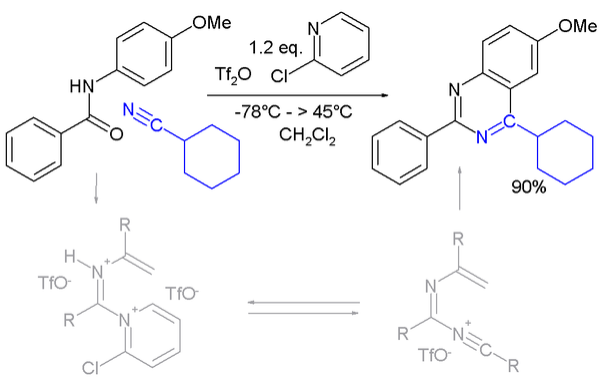
A novel method is by reaction of N-vinyl and N-aryl amides with carbonitriles under electrophilic activation of the amide with 2-chloro-pyridine and trifluoromethanesulfonic anhydride:
Uses
Pyrimidine is a heterocyclic organic molecule found in numerous pharmaceutical and naturally occurring compounds. It plays a significant role in the synthesis and discovery of antiviral medications, including those targeting HIV and HSV. Additionally, pyrimidine is involved in the synthesis of potent 15-lipoxygenase inhibitors that help reduce the release of leukotrienes. While pyrimidine itself has no direct applications, its derivatives, cytosine, uracil, and thymine, are essential components of nucleic acids. Various drugs, such as sulfadiazine, trimethoprim, and 6-mercaptopurine, also contain a pyrimidine ring.
Reactions
Electrophilic C-substitution of pyrimidine occurs at the 5-position, the least electron-deficient. Nitration, nitrosation, azo coupling, halogenation, sulfonation, formylation, hydroxymethylation, and aminomethylation have been observed with substituted pyrimidines.
Nucleophilic C-substitution should be facilitated at the 2-, 4-, and 6-positions but there are only a few examples. Amination and hydroxylation have been observed for substituted pyrimidines. Reactions with Grignard or alkyllithium reagents yield 4-alkyl- or 4-aryl pyrimidine after aromatization.
You may like
Related articles And Qustion
See also
Lastest Price from Pyrimidine manufacturers

US $6.00/kg2025-04-21
- CAS:
- 289-95-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $6.00/kg2025-03-28
- CAS:
- 289-95-2
- Min. Order:
- 1kg
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month