Difference between Benzyl chloride and chlorobenzene
What is Benzyl chloride?
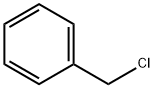
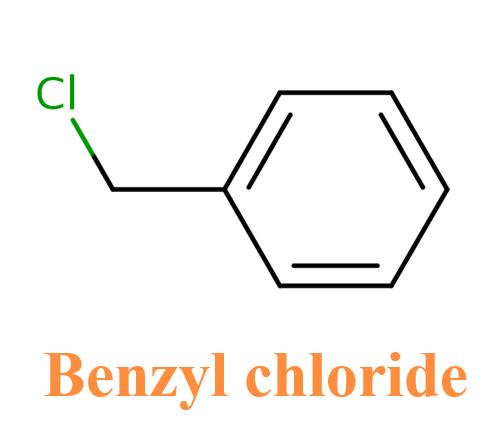
Benzyl chloride is an organobenzene compound with the chemical formula C₇H₇Cl. Its structure consists of a benzene ring, a methylene group, and a chlorine atom attached directly to the methylene group (-CH₂Cl). Appearance is colourless to light yellow liquid with strong unpleasant odour. Slightly soluble in water, density greater than water, irritating to the human body. It can be used as a chemical intermediate in the manufacture of certain dyes, pharmaceuticals (synthetic tannins), perfumes and flavouring products. It is also used as a photographic developer and as a gum inhibitor in petrol.
What is Chlorobenzene?
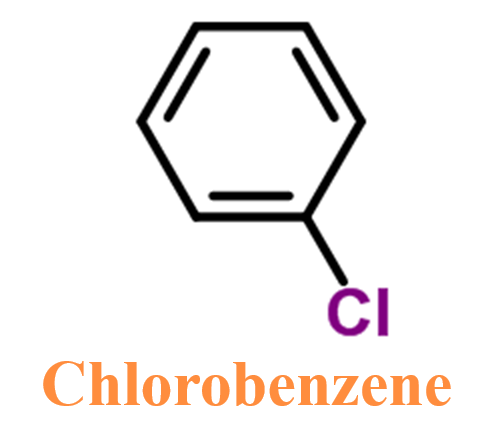
Chlorobenzene is also an organobenzene compound with the chemical formula C₆H₅Cl. Its structure consists of a benzene ring and chlorine atoms, but the chlorine atoms are directly attached to a carbon atom in the ring. It has the appearance of a clear, colourless liquid with a characteristic odour. It is also slightly soluble in water and is denser than water. It is used as a degreasing agent and as a solvent in various chemical reactions. It is also used in the production of other chemicals such as pesticides and dyes.
How do you distinguish between chlorobenzene from benzyl chloride?
Structurally Benzyl chloride and Chlorobenzene are both organic aromatic compounds with benzene rings and chlorine atoms in their structures. The difference is that Benzyl chloride also contains a methylene group and the position of the chlorine atom is slightly different. Benzyl chloride is more reactive than chlorobenzene due to the presence of a reactive chloromethyl group (-CH₂Cl), whereas chlorobenzene is more structurally stable and less prone to hydrolysis. This is due to the fact that chlorine is highly electronegative and the carbon-chlorine bond is very strong, so the carbon-chlorine bond requires a very high energy to break. In addition, phenol is an electron-withdrawing group, whereas chlorine is electronegative. Therefore, it will not be hydrolysed by NaOH. Since the attached chlorine atoms are not directly attached to the ring, it is easier to break the carbon-chlorine bond. Benzyl chloride can form a stable benzyl carbonyl intermediate, so it will have a chance of being hydrolysed. The carbonyl group formed is resonance stable.
Benzyl chloride is treated with potassium hydroxide or wetted silver oxide to produce benzyl alcohol. The reaction is as follows: C6H5CH2-Cl + KOH (aq) C6H5CH2OH + KCl.
Related articles And Qustion
See also
Lastest Price from Benzyl chloride manufacturers

US $1.00/KG2025-04-21
- CAS:
- 100-44-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1400.00/T2024-05-08
- CAS:
- 100-44-7
- Min. Order:
- 1T
- Purity:
- 98%
- Supply Ability:
- 20T