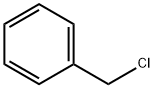
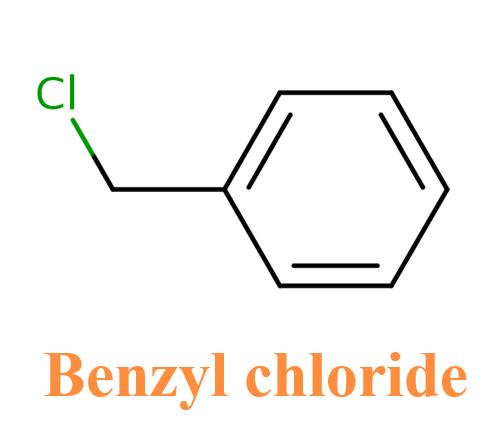
Benzyl chloride:Physical Properties and Chemical Properties
Benzyl chloride (chloromethylbenzene, a-chlorotoluene) [100-44-7] may be structurally the simplest side-chain chlorinated derivative of toluene, but economically it is the most important. Benzyl chloride is the starting material for a large number of industrial syntheses. The first preparation of it involved not the chlorination of toluene, however, but the reaction of benzyl alcohol with hydrochloric acid.

Physical Properties
Benzyl chloride is a colorless liquid which fumes in moist air. It has a pungent odor and is irritating to the mucous membranes and the eyes (i.e., it has a powerful lachrymatory effect).
The solubility of benzyl chloride in water is 0.33 g/L at 4 ℃, 0.49 g/L at 20 ℃, and 0.55 g/L at 30 ℃. Benzyl chloride is freely soluble in chloroform, acetone, acetic acid esters, diethyl ether, and ethyl alcohol. The solubility of chlorine in 100 g of benzyl chloride is 8.0 g at 30 ℃, 5.4 g at 50 ℃, and 2.1 g at 100 ℃.
Chemical Properties
Benzyl chloride can serve as a starting point for the preparation of benzal chloride and benzotrichloride, both of which are accessible by sidechain chlorination. Nuclear chlorination, on the other hand, leads to chlorobenzyl chlorides. Oxidation with sodium dichromate gives sodium carbonate in aqueous solution benzaldehyde and benzoic acid.
Metals undergo a variety of reactions with benzyl chloride. For example, magnesium in ether gives benzyl magnesium chloride (Grignard), whereas copper powder or sodium gives 1,2-diphenylethane as the main product (Wurtz synthesis). The action of Friedel–Crafts catalysts such as FeCl3, AlCl3, and ZnCl2 gives condensation products of the (C7H6)n type, but despite the fact that the degree of condensation can be controlled by changing the reaction conditions, these polymers have no commercial significance. If benzene or toluene is added to benzyl chloride in the presence of Friedel–Crafts catalysts, one obtains diphenylmethane or the isomeric benzyltoluenes, respectively.
The action of hydrogen sulfide and sulfides of the alkali metals leads to benzyl mercaptan and dibenzyl sulfide, respectively. Reactions with sodium salts of carboxylic acids produce the corresponding benzyl esters.
Hydrolysis with hot water is said to result in the formation of benzyl alcohol; nevertheless, this reaction has no industrial applications because the hydrochloric acid formed causes the reformation of benzyl chloride from the product benzyl alcohol; it also catalyzes the formation of dibenzyl ether.
Hydrolysis in the presence of alkali does lead to benzyl alcohol, however. Benzyl chloride reacts with sodium cyanide to give phenylacetonitrile (benzyl cyanide). Reaction with ammonia or amines gives primary, secondary, tertiary amines, and quaternary ammonium salts. The reaction with hexamethylenetetramine produces benzaldehyde (Sommelet reaction).
Related articles And Qustion
See also
Lastest Price from Benzyl chloride manufacturers

US $1.00/KG2025-04-21
- CAS:
- 100-44-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1400.00/T2024-05-08
- CAS:
- 100-44-7
- Min. Order:
- 1T
- Purity:
- 98%
- Supply Ability:
- 20T