Pyrimidine: anticancer activity
Introduction
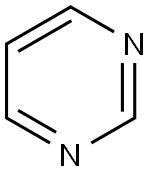
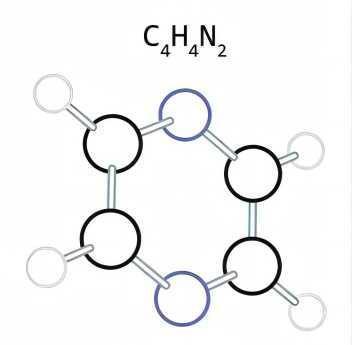
Cancer is a life-threatening ailment worldwide. Present treatment modalities like chemotherapy and radiotherapy suffers serious setbacks with multidrug resistance (MDR) being the major challenge. Search for diverse and novel structural framework may pave way to develop new effective anticancer drugs. Pyrimidine (1) is considered a vital heterocyclic moiety on account of its large spectrum of biological and pharmacological activities. These six-membered 1,3-diazine ring containing nitrogen at 1 and 3 position are part of naturally occurring substances such as nucleotides, nucleic acids, vitamins, coenzymes, purines, pterins, and uric acids. The widespread therapeutic applications of pyrimidine may be accounted for its presence in the structure of DNA and RNA. 5-halogenated derivatives of pyrimidine were among the first analogs tested for biological activity. This heterocyclic moiety is part of several drugs like zidovudine, stavudine, 5-flurouracil, methotrexate, imatinib, dasatinib, pazopanib, nilotinib, uramustine, tegafur, cytarabine, trimethoprim, sulfamethazine, minoxidil, phenobarbital, primidone, and risperidone. This review emphasizes advances over the last decades in pyrimidine containing hybrids with in vitro anticancer potential and its correlation with the SAR.[1]

2,4-Disubstituted pyrimidine derivative
Some novel 2,4-disubstituted pyrimidines were developed and tested for antiproliferative activity using MTT assay with VX-680 as positive control. Compound 2 was moderate to highly active against the A549 (IC50 = 12.05 ± 0.45 μM), HTC-116 (IC50 = 1.31 ± 0.41 μM), and MCF-7 (IC50 = 20.53 ± 6.13 μM) cell lines and exhibited potent aurora kinase inhibition against both aurora A and B kinase. Apoptosis was induced due to upregulation of Bax and downregulation in Bcl-xl. The SAR studies suggests that the benzene ring when replaced with cyclohexyl group gave better activity and replacement of NH in urea with CH2 lead to decrease in activity. It was established further that the blockade of G2-M phase of cell cycle occurred by accumulation of the contents at S phase due to decrease in mitochondrial membrane potential making 2,4-diaminopyrimidine derivatives potential anticancer agents. 3a (IC50 = 2.14 to 5.52 μM) and 3b (IC50 = 1.98 to 4.27 μM) were most potent against the PC-3, A549, MCF-7, and HCT-116 cancer cell lines due to the variation of substitution on aromatic ring and terminal aniline on the pyrimidine moiety. The induction of apoptosis was observed for cancer cell line K562 by novel anilino substituted pyrimidine sulfonamides. Cell viability was tested through MTT and tunnel assay. Compounds 4 demonstrated a promising activity with IC50 range = 5.6 to 12.3 μM.
2, 4, 6-Trisubstituted pyrimidines
Recently, in a study of synthesis and anticancer activity of trisubstituted pyrimidines and their N-alkyl derivatives was studied via ELISA, BRU, and MTT assay and 10 posed exceptional activity. They were tested against A549, Hep3B, HT29 FL, MCF-7, and HeLa cell lines with the IC50 range from 2 to10 μm/ml. Moreover, anthranillic acid ester moiety-linked 2,4,6-trisubstituted pyrimidines were tested for cytotoxic activty. The compounds were screened against U-937, CEM-13, MDA-MB-231, DU-145, and BT-474 cancer cell lines by conventional MTT assays. 11a and 11b were known to be the most potent in the series and also as CDK9 inhibitors. The SAR studies reveal that the major activity is due to the (E)-styryl moiety at C-6 position, methyl group at R2 position, and the presence of methylanthranilate moiety with an EDG at C-4 lead to better activity.[2]
Pyrimidines and triazolopyrimidines as antiproliferative agents exhibited COX-1/2 inhibitory potential. Compound 12 (IC50 range = 8.68 ± 0.2 to 36.56 ± 0.9 μg/ml) displayed in vitro activity against cancer cell lines HepG-2, MCF-7, CaCo-2, and A549 alongside COX-2 inhibition using 5-FU as the reference drug. Previously in a study, combretastatin bridged pyrimidine derivatives were tested for antitumor activity against the MCF-7 and A549 using MTT assay. 13a (IC50 = 4.67 μM; 3.38 μM) and 13b (IC50 = 0.63 μM; 3.71 μM) were concluded to have the best potential. 13a induced apoptosis by ROS-regulated intrinsic apoptotic pathway; they were non-toxic to harmful cells and were more potent inhibitors than cholchicne in the tunnel assay. The SAR demonstrated that the R2 and R3 substituted rings affected the activity, EWG such as 2,4-dichlorosubstitution on the rings manifested good activity, and interchange of amine, methyl with hydrogen in R1 position of the pyrimidine ring displayed no activity. Replacement of rings with napthyl gave less activity and no substitution in any of the three rings also depicted activity.
In a library of N-trisubstituted pyrimidine scaffold, compound 14 (IC50 = 12.2 nM) elucidated the best activity in the inhibition of U937 cell line. It caused the inhibition by inducing polyploidy (4N, 8N, and 16N) in the cancer cells by inducing defects in both chromosome formation and spindle formation. The SAR studies are depicted. In a continued study, a series of pyrimidine-benzimidazole compound 15 with an IC50 = 1.06 to 12.89 μM, was tested against the MGC-803, SMMC-7721, EC-9706, and MCF-7 cell lines antiproliferative activity. The cell cycle came to rest at G2/M phase by the active compound accompanied by an increase in apoptotic cell death of MGC-803. Additionally, in the previous year, the novel thiazolopyrimidine derivatives were studied against the human cancer cell lines and primary CLL cells. 16 displayed excellent anticancer activity against the cell lines and led to cell death by apoptosis as it inhibited the CDK enzyme.
Pyrazolo [1, 5-a]pyrimidines
In a recent report, some novel fused pyrazolo-pyrimidine derivatives were studied for anticancer activity as well as COX-2 inhibition against a 60 cancer cell line panel. Compound 29 was potent in case of both the studies. [3]It was selective towards COX due to the presence of 5-amino-1-oxo-substituted-pyrazole-4-carbonitrile moiety. Pyrazolo pyrimidine derivatives which were tested for cytotoxicity by the MTT assay against cancer cell lines PC-3, HCT116, and HepG-2. 30a (IC50 = 67.27 ± 3.8 μM/mL) and 30b (IC50 = 58.44 ± 3.8 μM/mL) demonstrated the best activity against HCT116 and PC-3 cell lines. SAR studies revealed that the order of antitumor activity was 4 methyl phenyl> 4 chloro phenyl> phenyl derivative against the cell lines and chlorine atom at 2 position was more active than 3 and 4 positions. In another study by the same group, antitumor activities of pyrazolo pyrimidines was screened against HepG-2 and MCF-7 using MTT assay. 31a (IC50 =63.2 ± 5.9 μg/mL) was reported have the best potential against MCF-7 carcinoma cells and 31b (IC50=70.3 ±4.1 μg/mL) against HepG2 carcinoma cells. SAR suggested that substitutions with bulky groups like methoxy and bromo gave significant antitumor activity. A new series of diamide substituted pyrazolo pyrimidine derivatives were reported. 32(a–c) were active against HeLa cell lines where IC50 value of each was less than 10 μM better than the marketed drug cisplatin. MTT assay was conducted to evaluate the cytotoxicity of the compounds.
References
[1] MohanaRoopan S, Sompalle R (2016) Synthetic chemistry of pyrimidines and fused pyrimidines: a review. Synth Commun 46(8):645–672
[2] Prachayasittikul S, Pingaew R, Worachartcheewan A, Sinthupoom N, Prachayasittikul V, Ruchirawat S, Prachayasittikul V (2017) Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini-Rev Med Chem 17(10):869–901
[3] Selvam TP, James CR, Dniandev PV, Valzita SK (2012) A mini review of pyrimidine and fused pyrimidine marketed drugs. Res Pharm 2(4):01–09
You may like
Related articles And Qustion
See also
Lastest Price from Pyrimidine manufacturers

US $6.00/kg2025-04-21
- CAS:
- 289-95-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $6.00/kg2025-03-28
- CAS:
- 289-95-2
- Min. Order:
- 1kg
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month