Quaternary Ammonium Compounds - Application in Meat Products and Food Industry
Characteristics and Properties
Quaternary ammonium compounds (QAC) are surface active agents (surfactants) commonly added to personal hygiene, pharmaceutical, food industry, and environmental sanitation products to act as antiseptics, disinfectants, conditioners, and emulsifiers (International Programme on Chemical Safety [IPCS], n.d.). QAC contain ampiphilic regions that interact with and disrupt/damage cell membranes, ultimately resulting in the loss of action potential and death (Oyarzabal, 2005) . Viruses, fungi, and bacteria are susceptible to QAC (Table 14.4 ), although gramnegative bacteria are less susceptible than gram-positive types especially at sublethal concentrations (Maxcy, Tiwari, & Soprey, 1971) . Maxcy et al. (1971) examined acquired QAC-tolerance in E. coli and found that tolerant cultures grew more slowly in growth medium and were more sensitive than parental cultures and unlikely to persist in the environment. The development of QAC-tolerance was dependent upon the structure and degree of affinity of each compound for microbial cells, as determined by adsorption, filtration, and elution of remaining QAC after treatment (Maxcy et al., 1971) .
Like many sanitizing solutions, the efficacy of QAC increases at higher temperatures, and the negative impact of organic matter on antimicrobial activity is enhanced at low temperatures (Table 14.7). Activity is reduced in the presence of metallic ions (hard water source) and optimal pH during application is food product- and microorganism-specific, although the highest level of activity is usually observed at acidic pH values (Cords et al., 2005) . QAC are not compatible with anionic surfactants/detergents or soaps and form a film with residual antimicrobial activity (Chmielewski & Frank, 2003) ; thus, fermentative lactic acid cultures may be inhibited in meat products previously treated with QAC compounds. Cetylpyridinium chloride (CPC) is a surface-active QAC commonly added to toothpaste, mouthwash, and cough drop formulations due to its ability to impede attachment of plaque-forming bacteria to tooth enamel (McDonnell & Russell, 1999 ; Oyarzabal, 2005) .
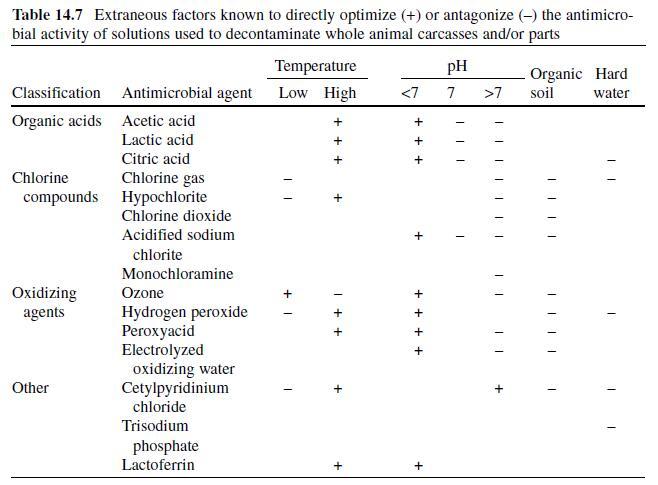
As derived from Axtell et al. (2006); Ayebah et al. (2005); Chmielewski and Frank (2003); El-Kest and Marth (1988); Hardin et al. (1995); Kaczur and Caulfield (1994); Leistner and Gouldm (2002); Moore, Griffiths, and Peters (2000); Naidu (2002); Prakash (2000); Restaino, Frampton, Hemphill, and Palnikar (1995); Russell and Axtell (2005); Schumb et al. (1955); Sofos Busta (1991); Stratford and Eklund (2003); Taylor, Rogers, and Holah (1999); White (1992); WHO (2004); Yang and Chen (1979).
Absence of symbol indicates negligible effect of a factor on the activity of the corresponding antimicrobial agent.