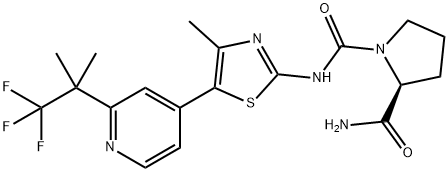
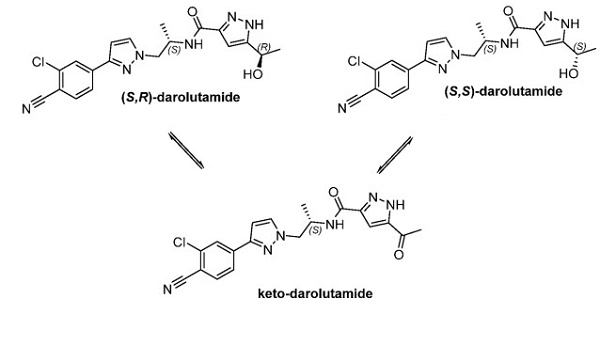
An orally PI3K inhibitor: Alpelisib
Description
Alpelisib -an orally available phosphatidylinositol 3-kinase (PI3K) inhibitor with specific activity against PI3K alpha (PI3Kα)-is being developed by Novartis for the treatment of breast cancer[1]. Alpelisib has demonstrated efficacy in combination with fulvestrant as a treatment for hormone receptor (HR)-positive, human epidermal growth factor receptor-2 (HER2)-negative breast cancer in patients with a PIK3CA mutation and was recently approved for this indication in the USA.
Properties
Alpelisib IC50 was 50 times lower for the α enzyme than for the β, δ and γ PI3K enzymes, leading to a decrease in intra-tumoral AKT phosphorylation. The PK properties of alpelisib are somehow favorable, with rapid and important absorption, a limited CYP P450-mediated metabolism, and a predominant biliary excretion, with a half-life of 17.5 ± 5.9 h. Only limited drug–drug interactions are expected, and there is no need for dose adaptation in mild and moderate renal impaired and mild to severe hepatic impaired patients. The main adverse events are hyperglycemia, rash, and diarrhea. The first, if not fully contra-indicated in (pre-)diabetic patients, warrants a close follow-up when treatment is started and a potential dose reduction when needed. Because of its safety profile, alpelisib requires stringent patient selection and close follow-up[2].
Synthesis method
Multiple syntheses of alpelisib have been reported, but no explicit process-scale approach has been disclosed[3]. Novartis reported a linear route that appears to be adaptable toward scale, and this approach is shown below. The subjection of picoline analog 233 to Ruppert's reagent (TMSCF3) in sodium acetate followed by hydrolytic silyl group removal provided tertiary alcohol 234 in quantitative yield. Mesylation of the tertiary alcohol proceeded in 85% yield by treatment with NaH and mild heating. Subsequent exposure to AlMe3 in cyclhexane provided intermediate 235, the picoline core of alpelisib, in 28% yield.
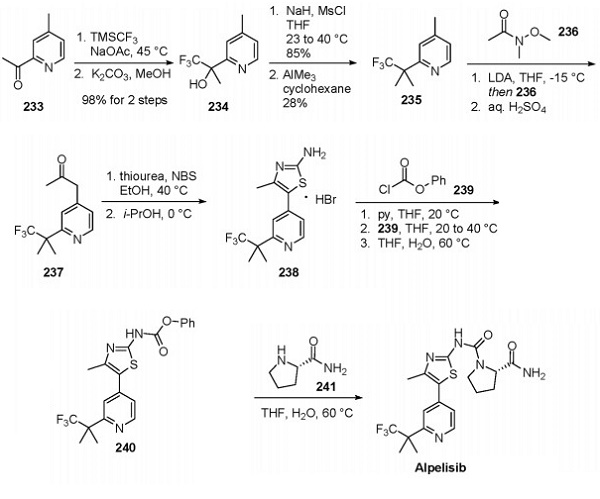
Lithiation of the benzylic methyl group at the 4-position and subsequent quench with Weinreb amide 236 provided 4-pyridylacetone 237 after an acidic workup. Treatment of 237 with thiourea in the presence of NBS produced 2-aminothiazole 238 as the HBr salt, precipitated from the solution by adding isopropanol. Neutralization of 238 with pyridine followed by reaction with phenyl chloroformate (239) yielded 2-aminothiazole carbamate 240. The phenoxy group was displaced upon installation of L-prolineamide (241), ultimately providing alpelisib.
References
[1] Markham, Anthony. “Alpelisib: First Global Approval.” Drugs 79 11 (2019): 1249–1253.
[2] Bernard Royer, Antonin Schmitt, Courèche Guillaume Kaderbhaï. “Pharmacokinetics and Pharmacodynamic of Alpelisib.” Clinical Pharmacokinetics 62 1 (2023): 45–53.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from Alpelisib (BYL719) manufacturers

US $2.00-5.00/kg2025-06-17
- CAS:
- 1217486-61-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00/G2025-04-21
- CAS:
- 1217486-61-7
- Min. Order:
- 1G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month