The introduction of Darolutamide (ODM-201)
Description
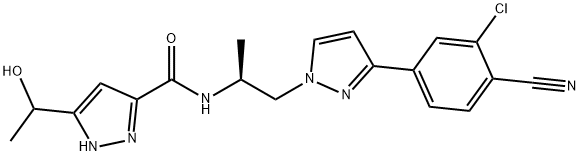
Darolutamide (ODM-201) is a structurally distinct non-steroidal androgen receptor (AR) antagonist being developed by Orion and Bayer as a treatment for prostate cancer. Based on positive results in the phase III ARAMIS trial, darolutamide was recently approved in the USA for the treatment of men with non-metastatic castration-resistant prostate cancer[1].
Property
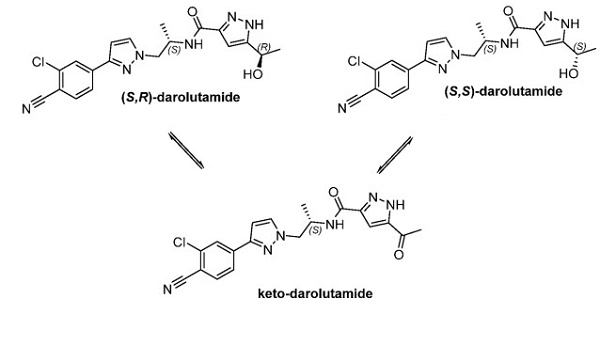
AR is a ligand-dependent transcription factor that controls the expression of various genes involved in cell growth and survival. Aberrant activation of AR is a well-known driver of prostate cancer. Second-generation AR antagonists, such as darolutamide, inhibit many of the AR variants that cause resistance to first-generation antagonists. Interestingly, as shown below, darolutamide is a mixture of two diastereomers that interconvert in vivo via oxidation to a ketone metabolite (keto-darolutamide) followed by reduction. The two alcohol diastereomers and the ketone metabolite have similar pharmacological activities.
Synthesis method
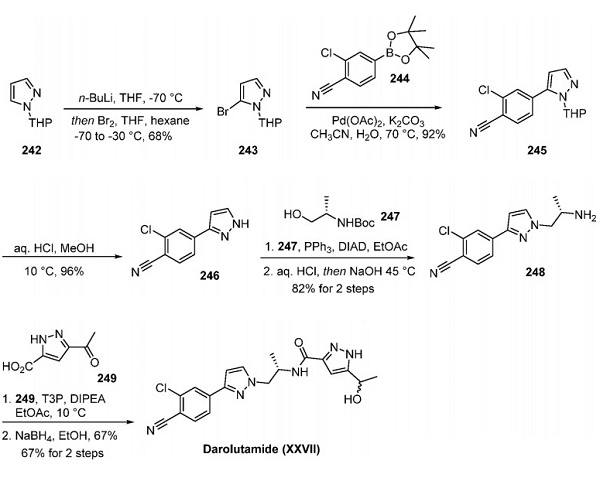
Pyrazole 242 was lithiated at C-5 and quenched with bromine to yield bromopyrazole 243, making way for coupling with pinacol borane 244 to afford nitrile 245. Removal of the THP protecting group from 245 with methanolic HCl gave rise to pyrazole 246 in 88% yield over two steps. Next, a Mitsunobu reaction between pyrazole 246 and alcohol 247 and subsequent Boc removal afforded amine 248 in 82% yield. As noted by Hughes, Mitsunobu reactions are rare in process chemistry because of the challenge of separating the reaction byproducts from the desired product. However, because protonated amine 248 was water-soluble, this product could be partitioned into the aqueous phase upon acidic workup, allowing the reaction byproducts to be retained in the organic phase and removed. The desired product was isolated in 82% yield after adjustment of the aqueous-phase pH and extraction. Amine 248 was then coupled with carboxylic acid 249 using T3P. Finally, the ketone was reduced with sodium borohydride in ethanol to produce the final product, Darolutamide, as a mixture of diastereomers in 67% yield over two steps[2].
Pharmacokinetics
Darolutamide had a steady-state mean Cmax and AUC12 of 4.79 mg/L and 52.82 h·μg/mL, respectively, after administration of repeated 600 mg twice daily doses; steady-state was achieved after 2–5 days, with≈2-fold accumulation. After administration of a single 600 mg oral dose of the drug, the time to reach Cmax (Tmax) was≈4 h. The bioavailability of darolutamide was increased 2.0–2.5-fold when given with food. Darolutamide and keto-darolutamide are 92% and 99.8% protein bound, respectively. Darolutamide is predominantly metabolized by CYP3A4 and, to a lesser extent, UGT1A9 and UGT1A1. It has also been identified as a substrate for P-gp and BCRP, according to preclinical data. The total exposure of keto-darolutamide in plasma is 1.7-fold higher than that of darolutamide.
References
[1] A. Markham, S. Duggan. “Darolutamide: First Approval.” Drugs 79 1 (2019): 1813–1818.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
Related articles And Qustion
See also
Lastest Price from ODM-201 manufacturers

US $0.00-0.00/KG2025-10-17
- CAS:
- 1297538-32-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500kg

US $1000.00-400.00/kg2025-04-21
- CAS:
- 1297538-32-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000