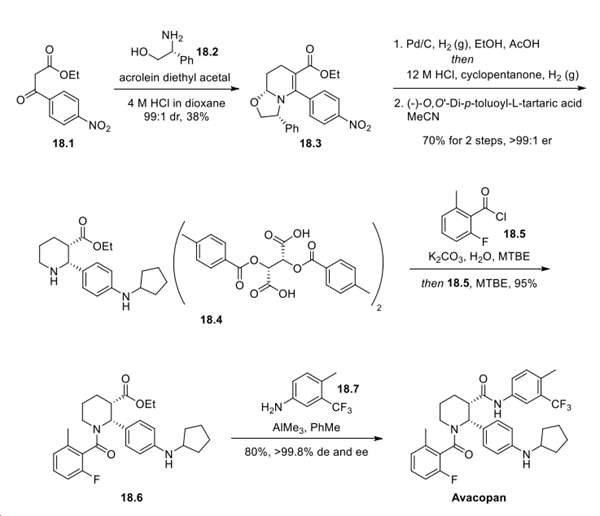
An antibody drug conjugate: Enfortumab Vedotin
Description
Enfortumab vedotin (EV) is an antibody−drug conjugate (ADC) consisting of a fully humanized monoclonal antibody targeting nectin-4 linked to the microtubule-disrupting agent monomethyl auristatin E (MMAE). These two entities are connected via a linker consisting of a maleimide conjugation handle that includes an enzyme-cleavable valine−citrulline−p-aminobenzyl alcohol group that releases MMAE after the conjugate has been internalized by the cancer cell recognizing the antibody.
Uses
Enfortumab vedotin could deliver monomethyl auristatin E (MMAE), a microtubule-disrupting agent, inside cells harboring the cell surface nectin-4 receptor[1]. The USFDA approved this drug for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma who have previously received a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor and platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced, or metastatic setting. Enfortumab vedotin was discovered at Agensys prior to the acquisition of Agensys by Astellas Pharma. EV is generally well tolerated, with the main toxicities being neuropathy, skin rash, alopecia, and fatigue. otably, EV can also be administered to patients with renal dysfunction, which is commonly a concern in this patient population. EV is now tested in combination strategies and earlier disease settings in urothelial cancers.
Synthesis method
Enfortumab vedotin was synthesized according to the methods shown above. The nectin-4 monoclonal antibody Ha22-2(2,4)6.1 (250) was dissolved in a buffer consisting of 10 mM acetate (pH 5.0), 1% sorbitol, 3% arginine, 20 vol % 0.1 M TrisCl (pH 8.4), 25 mM EDTA, and 750 mM NaCl that was adjusted to pH 7.5. This solution was treated with tris(2-carboxyethyl)phosphine (TCEP) to reduce the intrachain disulfide bonds to give intermediate 251. The reaction of the free thiol groups with 4.4 equiv of the linker-payload mc-ValCitPABC-MMAE (252) followed by reoxidation of the remaining disulfide bonds and quenching of the remaining linker−payload with N-acetylcysteine provided enfortumab vedotin with a drug to antibody ratio (DAR) of 3.8. Linker−payload 252 is commercially available and was first used to synthesize brentuximab vedotin (Adcetris)[2].
References
[1] Alt, Marie , et al. "Enfortumab Vedotin in urothelial cancer." Therapeutic Advances in Urology 12(2020): 175628722098019.
[2] Flick, Andrew C. , et al. "Synthetic Approaches to the New Drugs Approved during 2019.".