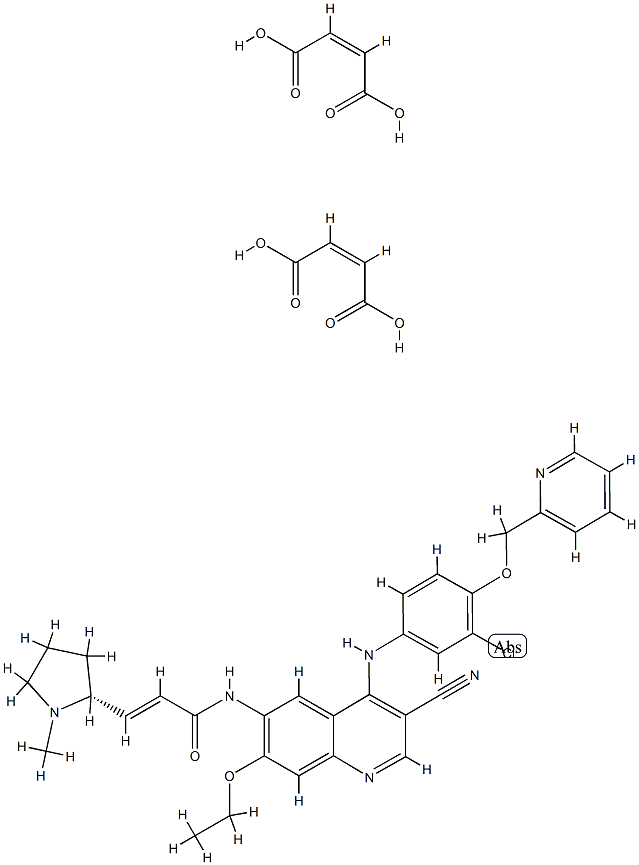
An irreversible EGFR/HER2 dual tyrosine kinase inhibitor: Pyrotinib Maleate
Description
Pyrotinib, an irreversible EGFR/HER2 dual tyrosine kinase inhibitor used for treating HER2-positive breast cancer, is primarily metabolized by cytochrome P450 (CYP)3A4 isozyme[1]. Pyrotinib maleate, an oral irreversible pan-ErbB receptor tyrosine kinase inhibitor (TKI) developed by Hengrui Pharmaceutical, received conditional approval in China in August 2018 for the treatment of HER2-positive, advanced, or metastatic breast cancer in combination with capecitabine in patients previously treated with anthracycline or taxane chemotherapy.
Pyrotinib and neratinib
Structurally, pyrotinib is similar to neratinib, which is also approved for treating HER2-positive breast cancer, differing in terminal amine substitution of the Michael acceptor covalent-binding warhead. An account of the discovery and development of pyrotinib, focused primarily on modifications of the left-side 2- butenoyl side chain of neratinib, has been published. The terminal methylpyrrolidine group within pyrotinib demonstrated improvements in cellular potency, favorable PK profiles, in vivo antitumor efficacy, and safety profile.
Synthetic method
The process-scale synthesis of cyanoquinoline 347, a commercially available intermediate for pyrotinib and neratinib, likely follows the route to neratinib published by scientists from Wyeth, as described in an earlier edition of this annual review. From aminoquinoline 347, acylation to introduce the side chain rapidly provided access to pyrotinib freebase (349, Scheme 1)[2]. A patent from Hengrui describes two kilogram-scale approaches to this acylation: (1) amidation of acid 348 with EEDQ in NMP and (2) via the corresponding acid chloride of compound 348. While the acid chloride route required column chromatography, the EEDQ conditions allowed for chromatography-free isolation of pyrotinib in 80% yield and high purity following acid/base extraction and crystallization from ethanol and acetone. Subsequent treatment with maleic acid in ethanol and crystallization from diethyl ether provided pyrotinib maleate with a 79% yield.
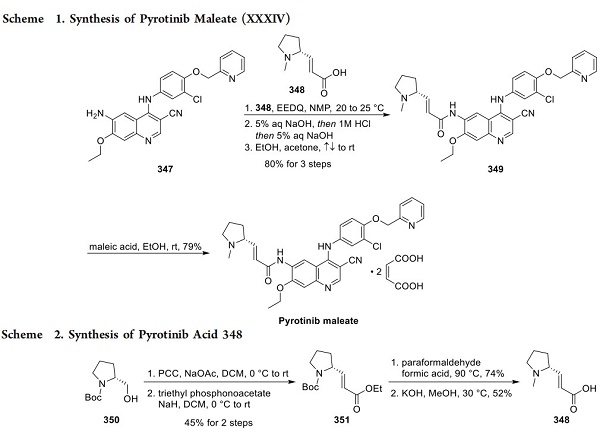
Acid 348 was generated on kiloscale in four steps from NBoc-D-prolinol (350, Scheme 2). Following oxidation to the corresponding aldehyde, a Horner−Wadsworth−Emmons (HWE) reaction with triethyl phosphonoacetate gave α,β- unsaturated ester 351 in 45% yield. Reduction of N-Boc to NMe in the presence of paraformaldehyde and formic acid followed by ester hydrolysis generated acid 348.
References
[1] Ming-min Cai. “Effects of rifampicin on antineoplastic drug pyrotinib maleate pharmacokinetics in healthy subjects.” Investigational New Drugs 40 1 (2022): 756–761.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.