Microbial Fermentation and Biosynthesis Pathways: L-Malic Acid Production in Multiple Industries
General Description
L-Malic acid, a key ingredient in various industries, can be produced through microbial fermentation or chemical conversion. Microorganisms can directly synthesize L-malic acid from glucose, convert fumaric acid to L-malic acid using fumarase, or produce precursor PMA which can be hydrolyzed into L-malic acid. The non-oxidative pathway involves carboxylation of pyruvate to oxaloacetate and reduction to malate, while the oxidative pathway involves condensation of oxaloacetate and acetyl-CoA to form citric acid, which is then oxidized to malate through the TCA cycle. Factors such as enzyme expression and pH regulation play important roles in optimizing malic acid biosynthesis. These processes offer a cost-effective and environmentally friendly alternative to traditional extraction methods.

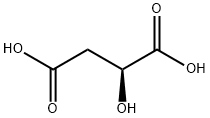
Figure 1. L-Malic acid
Production by microorganisms
L-Malic acid is a key ingredient in several industries such as food, medicine, and cosmetics due to its unique properties. Although extraction from fruits or eggshells is possible, it is not economical due to low yields and high energy consumption. Alternatively, L-malic acid can be produced through microbial fermentation or chemical conversion. One-step fermentation or catalysis of fumarase can convert pyruvic acid or fumaric acid to L-malic acid. Additionally, the acid hydrolysis of PMA can yield L-malic acid. Three microbial groups are capable of producing L-malic acid. The first group synthesizes L-malic acid directly from glucose via one-step fermentation. The second group converts fumaric acid to L-malic acid using pure fumarase or fumarase in its cells. The third group synthesizes PMA, which can be hydrolyzed into L-malic acid. These processes are cost-effective and environmentally friendly compared to traditional extraction methods, making them attractive alternatives for L-malic acid production. 1
Biosynthesis pathways
Non-oxidative pathway
The non-oxidative pathway for L-Malic acid biosynthesis involves the carboxylation of pyruvate to oxaloacetate, followed by reduction to malate. This pathway is found in various microorganisms such as S. cerevisiae, A. flavus, A. oryzae, E. coli, B. subtilis, and Penicillium spp. When pyruvate is produced during glycolysis, this pathway is ATP neutral and results in the fixation of CO2, allowing for a maximum theoretical malate yield of 2 mol per mol of consumed glucose. In practice, however, the actual yield is lower due to glucose being transformed into CO2 through respiration and cell mass through growth. Additionally, some glucose may be converted into other organic acids like citric acid, succinic acids, and fumaric acid. The involvement of pyruvate carboxylase, phosphoenolpyruvate (PEP) carboxylase, and malate dehydrogenase in malic acid biosynthesis has been suggested. Pyruvate carboxylase catalyzes the carboxylation of pyruvic acid, while malate dehydrogenase facilitates the reversible conversion of L-malic acid to oxaloacetic acid using NAD(H). Biotin and NAD(H) are essential cofactors for malic acid biosynthesis. Maintaining a near-neutral pH, achieved by adding CaCO3, is crucial for efficient malic acid production. Increased calcium concentrations have a positive effect on malate formation. Overexpressing fumarase or malate dehydrogenase can also enhance malic acid production in the oxidative and reductive tricarboxylic acid cycles respectively. 2
Oxidative pathway
The oxidative pathway for L-Malic acid biosynthesis involves the condensation of oxaloacetate and acetyl-coenzyme A (acetyl-CoA) to form citric acid, which is then oxidized to malate through the tricarboxylic acid (TCA) cycle. If acetyl-CoA is generated by pyruvate dehydrogenase, the conversion of glucose to malate via this pathway releases two CO2, limiting the maximum theoretical malate yield to 1 mol per mol of glucose. Fumarase, which facilitates the reversible hydration of fumaric acid to L-malic acid, plays a crucial role in malate formation within this pathway. Microbial cells carrying fumarase have been widely utilized for the transformation of fumaric acid to L-malic acid. In S. cerevisiae, overexpression of fumarase or malate dehydrogenase in the oxidative or reductive TCA cycle of mitochondria respectively enhances malic acid production. A respiratory-deficient strain of S. cerevisiae has been found to produce more L-malic acid compared to its parent strain. However, overexpressing the malic enzyme, responsible for the conversion of malic acid to pyruvic acid in mitochondria, leads to a decrease in malic acid production. These findings demonstrate the importance of fumarase and proper regulation of enzyme expression in optimizing malic acid biosynthesis through the oxidative pathway. 3
Reference
1. Lin S, Wang L, Jones G. Optimized extraction of calcium malate from eggshell treated by PEF and an absorption assessment in vitro. Int J Biol Macromol, 2012, 50, 1327–1333.
2. Zelle RM, de Hulster E, van Winden WA, et al. Malic acid production by Saccharomyces cerevisiae: engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl Environ Microbiol, 2008, 74, 2766–2777.
3. Nakayama S, Tabata K, Oba T, et al. Characteristics of the high malic acid production mechanism in Saccharomyces cerevisiae sake yeast strain No. 28. J Biosci Bioeng, 2012, 114, 281–285.
Related articles And Qustion
Lastest Price from L-Malic acid manufacturers

US $1200.00-1100.00/ton2025-08-06
- CAS:
- 97-67-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M
US $1.00-4.00/KG2025-06-24
- CAS:
- 97-67-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG