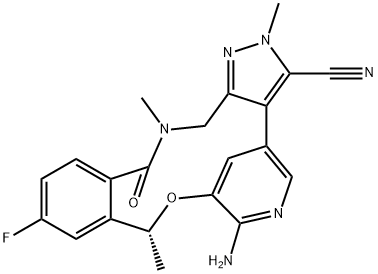
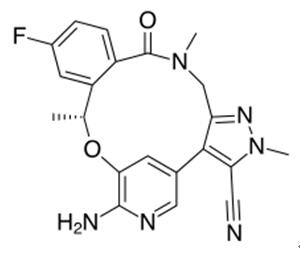
How to synthesize Lorlatinib?
Description
Lorlatinib is an oral small molecule inhibitor of anaplastic lymphoma kinase (ALK) and C-ros oncogene 1 (ROS1) kinase developed by Pfizer for the treatment of ALK-positive non-small cell lung cancer (NSCLC)[1]. Tumor progression emerges due to both brain metastases and resistance mutations, with the ALK G1202R mutant being particularly challenging to address with first and second-generation therapies. It is a macrocyclic ALK and cROS inhibitor that readily penetrates the blood−brain barrier and demonstrates significant activity against multiple ALK mutations, including G1202R, demonstrating the ability to address both issues.
Synthetic method
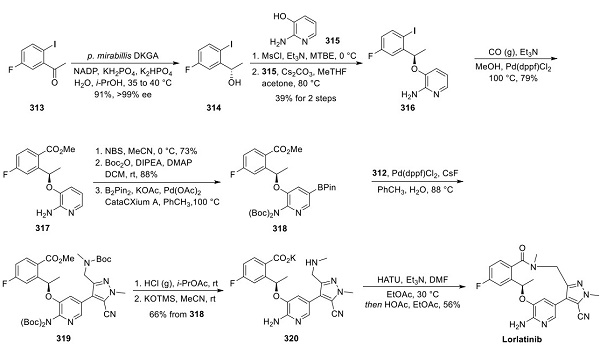
Preparation of Lorlatinib Aminopyrazole Fragment 312
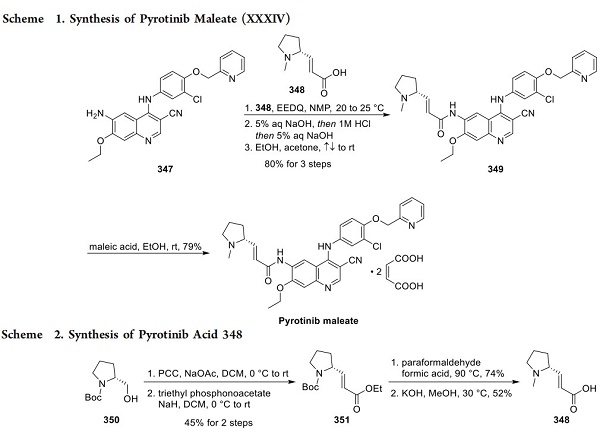
Structurally, lorlatinib presents considerable challenges with respect to the efficient construction of the macrocycle. The reported largest scale synthesis utilizes a convergent approach, demonstrated by a Suzuki cross-coupling to afford a penultimate intermediate and subsequent amide macrolactamization to yield the core structure[2]. This approach incorporates many key transformations and bond disconnections developed in the discovery synthesis, with subsequent optimization used to produce kilogram quantities efficiently. A gram-scale synthesis of the aminomethyl pyrazole fragment commenced with bis-bromination of pyrazole ester 308 (shown below). Protected methylamine equivalent 310 was introduced by treatment with sodium hydride and subsequent addition to 309 to yield intermediate 311. Conversion of ester 311 to nitrile 312 was accomplished using a high-yielding, three-step sequence employing ester hydrolysis, conversion of the resulting acid to a primary amide, and finally, dehydration of the primary amide to nitrile 312.
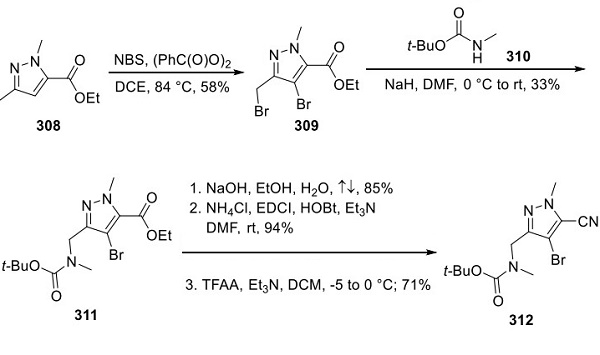
Synthesis of Lorlatinib
Enantioselective reduction of commercial ketone 313 was explored with various chemical reagents (CBS reduction, DIP-Cl). However, a biocatalytic solution was ultimately identified by screening a diverse set of ketoreductases. After optimization, the reduction proceeded in high yield and essentially complete enantioselectivity on >200 kg to yield 314. The critical pyridine ether bond was formed by activating the chiral secondary alcohol as the methanesulfonate ester. This was followed by SN2 displacement with 2-amino-3- hydroxypyridine (315) to afford 316. Carbonylation of the aryl iodide to methyl ester 317 was affected by palladium in the presence of carbon monoxide and MeOH. To generate the Suzuki coupling precursor, 317 was brominated on the pyridine para to the amino group, followed by bis-Boc protection, and then Miyaura borylation to give 318. Although Suzuki coupling of 318 and 312 was plagued by homocoupling of 318, slow addition of 318 in a toluene solution to a solution of 312, palladium catalyst, and CsF in an aqueous toluene mixture at reflux suppressed the side reaction to <3%, enabling high yields of 319. Acidic removal of the three Boc groups, followed by neutralization and nitrile-sparing ester hydrolysis with KOTMS, led to the precipitation of 320 as a crystalline solid. Macrocyclic amidation was affected by the slow addition of a DMF solution of 320 over 14 h into a dilute solution of HATU and triethylamine in EtOAc, which served to minimize intermolecular coupling. Following aqueous workup, treatment with acetic acid led to crystallization and isolation of the acetic acid solvate of lorlatinib in 56% yield and >99.2% purity.
References
[1] Syed, Yahiya Y. “Lorlatinib: First Global Approval.” Drugs 79 1 (2019): 93–98.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
Related articles And Qustion
Lastest Price from Lorlatinib manufacturers

US $1.00/kg2025-07-21
- CAS:
- 1454846-35-5
- Min. Order:
- 1kg
- Purity:
- 99.99%+
- Supply Ability:
- 100ton

US $0.00-0.00/mg2025-05-26
- CAS:
- 1454846-35-5
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000