Lorlatinib (PF-06463922): Overview and Therapeutic Trials in Non-Small Cell Lung Cancer
General Description
Lorlatinib (PF-06463922), developed by Pfizer, is a third-generation oral ATP-competitive inhibitor targeting ALK and ROS1 receptor tyrosine kinases, showing promise in treating ALK-positive and ROS1-positive non-small cell lung cancer (NSCLC). It overcomes the limitations of first-generation TKIs like crizotinib by effectively penetrating the blood-brain barrier and surpassing resistant mutations. Clinical trials have demonstrated its efficacy across various patient groups, with notable overall and intracranial response rates, particularly in crizotinib-naive patients for ROS1-positive NSCLC. Lorlatinib (PF-06463922) offers significant disease control, rapid tumor response, and potential quality of life improvements, marking a significant advancement in NSCLC therapy despite concerns about resistance development.

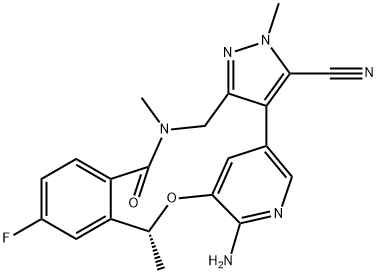
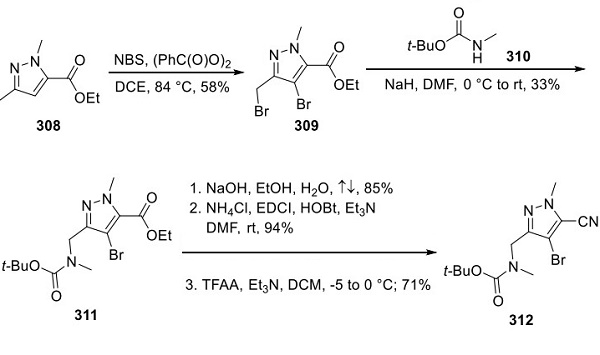
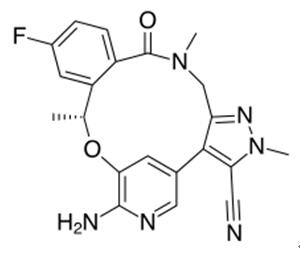
Figure 1. Lorlatinib (PF-06463922)
Overview
Lorlatinib (PF-06463922), developed by Pfizer, is an oral ATP-competitive inhibitor targeting ALK and ROS1 receptor tyrosine kinases, crucial for treating ALK-positive non-small cell lung cancer (NSCLC). It addresses the limitations of first-generation tyrosine kinase inhibitors (TKIs) like crizotinib, which, despite being effective, often lead to acquired resistance and insufficient brain penetration. Lorlatinib (PF-06463922) stands out as a third-generation TKI designed to surpass resistant ALK mutations and effectively cross the blood-brain barrier, enhancing treatment efficacy for patients with brain metastases. Approved in Japan in September 2018 and granted accelerated approval by the US FDA in November 2018, Lorlatinib (PF-06463922) offers a promising option for patients with ALK-positive metastatic NSCLC who have shown progression on previous ALK inhibitors or those intolerant to such treatments. Its approval underscores a significant step forward in NSCLC therapy, particularly for those with challenging ALK fusion gene-positive cases, although the potential for developing resistance to Lorlatinib (PF-06463922) remains a concern. 1
Therapeutic Trials in Non-Small Cell Lung Cancer
ALK-positive non-small cell lung cancer
Lorlatinib (PF-06463922) has been evaluated in therapeutic trials for ALK-positive non-small cell lung cancer demonstrating varying degrees of efficacy across different patient groups. In treatment-naive patients, the overall response rate (ORR) was 90%, with an intracranial ORR of 66.7%. Patients previously treated with crizotinib, with or without chemotherapy, showed an ORR of 69.5% and an intracranial ORR of 87%. Those who received prior non-crizotinib ALK tyrosine kinase inhibitors (TKIs) ± chemotherapy had lower ORRs of 32.1% and an intracranial ORR of 55.6%. Patients with ≥2 prior ALK TKIs ± chemotherapy experienced an ORR of 38.7% and an intracranial ORR of 53.1%. A pooled analysis of patients who had received ≥1 prior ALK TKI ± chemotherapy showed an ORR of 47% and an intracranial ORR of 63%. The median time to first tumor and intracranial response was notably rapid across all cohorts, with a median duration of intracranial response reaching up to 14.5 months in some groups. Lorlatinib (PF-06463922) was particularly effective in patients harboring ALK kinase domain mutations, including the challenging G1202R mutation, inducing a tumor response in 64% of these patients. Progression-free survival varied, with a median of 5.5 to 7.3 months in most cohorts, indicating significant disease control. Patient-reported outcomes revealed improvements or stability in global quality of life scores and functioning domain scores for a majority of patients. Symptoms such as fatigue, insomnia, appetite loss, pain, and dyspnoea improved in more than 25% of patients during Lorlatinib (PF-06463922) treatment, although cognitive functioning and peripheral nephropathy worsened in some cases. These results underscore Lorlatinib (PF-06463922) 's potential as a highly effective treatment option for ALK-positive NSCLC, offering meaningful clinical benefits and improvements in quality of life. 2
ROS1-positive non-small cell lung cancer
Lorlatinib (PF-06463922) has been explored in therapeutic trials for ROS1-positive non-small cell lung cancer, with varying outcomes based on prior treatment exposure. The overall response rates (ORRs) and intracranial ORRs in the total EXP6 cohort of 47 patients were 36.2% and 56.0%, respectively. Among crizotinib-naive patients (n=13), the ORR was notably higher at 61.5%, with an intracranial ORR of 66.7%. In contrast, patients previously exposed to crizotinib (n=34) showed a lower ORR of 26.5% and an intracranial ORR of 52.6%. The median progression-free survival (PFS) for these groups was 9.9 months for the total cohort, 21.0 months for crizotinib-naive patients, and 8.5 months for crizotinib-pre-exposed patients. These findings indicate that Lorlatinib (PF-06463922) is effective in treating ROS1-positive NSCLC, particularly in patients who have not been previously treated with crizotinib. The significantly longer PFS in crizotinib-naive patients underscores the potential for Lorlatinib (PF-06463922) as a first-line treatment option for this subset. The therapeutic trials, conducted across various phases and locations, including multinational and specific sites in the USA and Italy, highlight Lorlatinib (PF-06463922)'s role in managing ROS1-positive NSCLC, offering hope for improved outcomes in this challenging patient population. 2
Reference
1. Zou HY, Li Q, Engstrom LD, et al PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci USA. 2015;112(11):3493-3498.
2. Syed YY. Lorlatinib: First Global Approval. Drugs. 2019;79(1):93-98.
You may like
Related articles And Qustion
See also
Lastest Price from Lorlatinib manufacturers

US $1.00/kg2025-07-21
- CAS:
- 1454846-35-5
- Min. Order:
- 1kg
- Purity:
- 99.99%+
- Supply Ability:
- 100ton

US $0.00-0.00/mg2025-05-26
- CAS:
- 1454846-35-5
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000