Suvorexant: Mechanism of Action and Clinical Studies
General Description
Suvorexant, a dual orexin receptor antagonist, targets the brain's wakefulness system by inhibiting orexin A and B neurotransmitters, facilitating sleep initiation and maintenance. Its unique mechanism offers an alternative to traditional hypnotics, avoiding common side effects like dependency. Extensive clinical trials across Phase I to III have demonstrated suvorexant's safety, tolerability, and efficacy in treating insomnia in both non-elderly and elderly adults. These studies revealed dose-dependent pharmacokinetics and significant improvements in sleep efficiency and maintenance. Consequently, suvorexant has emerged as a promising, novel treatment option for insomnia, enhancing our understanding of sleep regulation and therapeutic interventions.

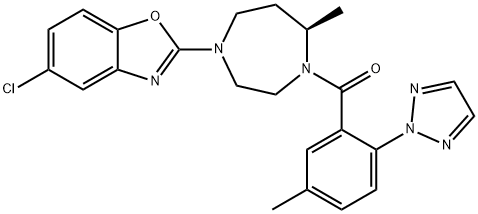
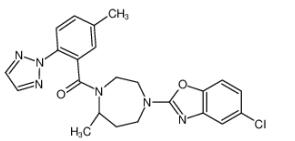
Figure 1. Suvorexant
Mechanism of action
Suvorexant operates as a dual orexin receptor antagonist (DORA), targeting the brain's wakefulness-regulating system. The mechanism of Suvorexant involves selectively inhibiting the actions of orexin A and B neurotransmitters, which are exclusively produced by neurons in specific hypothalamic areas. These neurotransmitters play a crucial role in promoting wakefulness by activating orexin 1 and 2 receptors located throughout the brain, notably within the monoamine neurotransmitter centers in the brainstem. Orexin A interacts with both types of receptors, while orexin B primarily targets orexin 2 receptors. The orexin 2 receptors, highly expressed in the tuberomammillary nucleus (TMN), are pivotal in mediating the effect of orexins on wakefulness, largely through the activation of TMN histaminergic neurons. By blocking both orexin 1 and 2 receptors, suvorexant effectively reduces the stimulation of these wakefulness-promoting pathways, facilitating the initiation and maintenance of sleep. This action distinguishes suvorexant from traditional hypnotics, such as benzodiazepines or Z drugs, by offering a novel approach to treating insomnia without their common side effects, including dependency, withdrawal symptoms, and cognitive impairments. 1
Clinical studies
Suvorexant, an orexin receptor antagonist, has undergone extensive clinical evaluation to assess its safety, efficacy, and pharmacokinetic profile for the treatment of insomnia. Phase I studies involving over 32 trials provided initial insights into suvorexant's safety, tolerability, and pharmacodynamics, including assessments of its potential for abuse, effects on respiratory safety, and impact on activities such as car driving. These studies, which included both non-elderly (18 to <65 years) and elderly patients (65 years or older), demonstrated suvorexant’s efficacy and safety, paving the way for further development in treating primary insomnia. Notably, no studies were conducted in children or infants. The pharmacokinetics of suvorexant, as reported by Sun et al., showed dose-dependent increases in the area under the curve (AUC) and peak concentration (Cmax), though not linearly. Despite suvorexant's long half-life and the time to reach maximum plasma concentration (Tmax) being 3 hours, it was found to promote sleep effectively in healthy young men, albeit with evidence of residual effects at higher doses. Phase II research, particularly the dose-ranging clinical trial (MK4305-P006), aimed to establish proof of concept for suvorexant's efficacy and safety in patients meeting DSM-4 criteria for primary insomnia. This multicenter, randomized, double-blind, placebo-controlled trial identified efficacious doses by demonstrating significant improvements in sleep efficiency, sleep maintenance, and sleep onset across various doses compared to placebo. Phase III studies further validated suvorexant's effectiveness and safety in a broader patient population, including both non-elderly and elderly adults. Pivotal dose-finding studies (P028 and P029) along with a long-term safety study (P009) showcased suvorexant's ability to improve sleep maintenance and latency metrics significantly. These studies, unique for their duration and comprehensive assessments, confirmed suvorexant as a promising treatment for insomnia, with consistent benefits observed over months of treatment. In summary, clinical research on suvorexant across different phases has thoroughly evaluated its pharmacokinetic profile, safety, and efficacy in treating insomnia. The findings from these studies have established suvorexant as a valuable option for managing insomnia, contributing to our understanding of its role in sleep regulation and reinforcing its therapeutic potential. 2
Reference
1. Stahl SM. Mechanism of action of suvorexant. CNS Spectr. 2016;21(3):215-218.
2. Jacobson LH, Callander GE, Hoyer D. Suvorexant for the treatment of insomnia. Expert Rev Clin Pharmacol. 2014;7(6):711-730.
Related articles And Qustion
See also
Lastest Price from Suvorexant manufacturers

US $0.00/g2024-11-19
- CAS:
- 1030377-33-3
- Min. Order:
- 1g
- Purity:
- 98% HPLC
- Supply Ability:
- 100kg

US $48.00-40.00/KG2023-05-04
- CAS:
- 1030377-33-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20tons