Synthetic route to the herbicide Fenquinotrione
Synthesis of Fenquinotrione
Fenquinotrione is synthesised using 2,6-Dichloronitrobenzene as a raw material by chemical reaction. The specific synthesis steps are as follows:
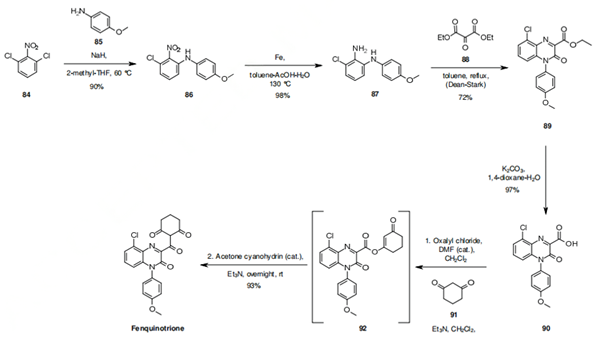
The synthesis of fenquinotrione involves the now well established O-Acyl coupling of the acid 90 to cyclohexandione (91), followed by a cyanide catalyzed rearrangement (via the acyl cyanide) of 92 to the C-acylated product. The required acid 90 for this chemistry is synthesized by an aromatic substitution reaction of 84 with 85 to give 86. Béchamp reduction of the nitro group, followed by a Dean-Stark condensation of 87 with diethyl ketomalonate (88) gives the ester 89. The reaction is regioselective, with the primary aniline function of 87 attacking the keto function of 88, with the secondary aniline function then attacking one of the ester carbonyls to close the ring. The isomer that would be obtained by the primary aniline 87 first attacking an ester functionality, and the secondary aniline then attacking the carbonyl, is not reported. Hydrolysis of the ester 89 with potassium carbonate and water gives the required acid 90 that can be converted to fenquinotrione by the rearrangement chemistry previously mentioned.
Introduction of Fenquinotrione
Fenquinotrione is a new herbicide developed by Kumiai Chemical Industry and Ihara Chemical Industry for use in rice and other crops. Fenquinotrione controls a wide range of sedges and broadleaf weeds with residual activity, and has excellent rice selectivity in any rice production system. Fenquinotrione exerts its herbicidal effect through inhibition of HPPD, and is effective on troublesome acetolactate synthase (ALS) resistant weeds, which are widely common in rice production areas.
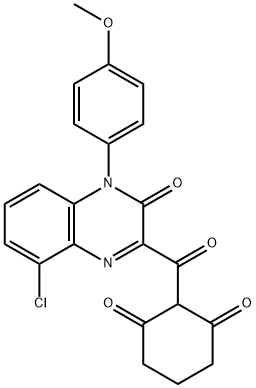 1,3-Cyclohexanedione, 2-[[8-chloro-3,4-dihydro-4-(4-methoxyphenyl)-3-oxo-2-quinoxalinyl]carbonyl]-
1,3-Cyclohexanedione, 2-[[8-chloro-3,4-dihydro-4-(4-methoxyphenyl)-3-oxo-2-quinoxalinyl]carbonyl]- 1342891-70-6

