What is Suvorexant?
Feb 10,2020
2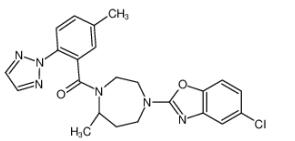

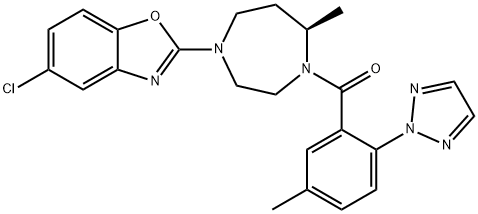
Suvorexant (BelsomraR), a first-in-class, orally active dual orexin-1 receptor and orexin-2 receptor antagonist, has been developed by Merck for the treatment of insomnia.
Suvorexant binds with high affinity to the human orexin-1 and orexin-2 receptors in vitro; this binding is > 6,000-fold more selective than that to more than 170 other known receptors and enzymes, with >90 % receptor occupancy. In several animal studies, suvorexant modulated the endogenous orexin signalling response and induced rapid sleep, with increases in both non-rapid eye movement and rapid eye movement sleep.[1]
In fasting conditions, the median time to maximum plasma concentration of suvorexant is 2 h. For suvorexant 10 mg, the mean absolute bioavailability is 82 %. Administration of suvorexant with a high-fat meal had no clinically significant on systemic drug exposure, but delayed tmax by 1.5 h. Steady state is achieved within 3 days. The mean volume of distribution of suvorexant is 49 L. Suvorexant is extensively bound to plasma proteins, including albumin and a1-acid glycoprotein, and does not preferentially distribute into the red blood cells. [2]
Suvorexant is primarily metabolized by CYP3A, with a minor contribution by CYP2C19. The main circulating compounds are the unchanged drug and a hydroxyl metabolite, which is not expected to be pharmacologically active. Suvorexant is primarily eliminated in the faeces. [3]
The recommended dosage is 10 mg, to be taken orally no more than once per night, within 30 min of going to bed and with at least 7 h remaining until the planned awaken time. If this dosage is well tolerated but not effective, the dosage can be increased up to a maximum of 20 mg once daily; the lowest effective dosage should be used. Patients taking concomitant moderate cytochrome P450 (CYP) 3A4 inhibitors should receive a reduced dose of 5 mg. [4]
Suvorexant was approved by the US FDA in August 2014 for the treatment of adult patients with insomnia characterized by difficulties with sleep onset and / or sleep maintenance. The drug is also preregistration in Japan, with approval submissions planned for other countries worldwide for this indication.
References
1.Mieda M, Sakurai T. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders: rationale for development and current status[J]. CNS Drugs. 2013, 27(2):83–90.
2.Connor K, Budd K, Snavely D, et al. Efficacy and safety of suvorexant, an orexin receptor antagonist, in patients with primary insomnia: a 3-month phase 3 trial (trial #1) [J]. J Sleep Res. 2012, 21(Suppl 1):97.
3.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant[J]. Neurology. 2012, 79(23):2265–74.
Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant: a novel dual orexin receptor antagonist[J]. J Neurogenet. 2011, 25(1–2):52–61.
You may like
Clinical Application Research of Sertraline
Dec 19, 2025
Application research of Ceftiofur hydrochloride
Dec 18, 2025
Related articles And Qustion
Lastest Price from Suvorexant manufacturers
Suvorexant

US $0.00/g2024-11-19
- CAS:
- 1030377-33-3
- Min. Order:
- 1g
- Purity:
- 98% HPLC
- Supply Ability:
- 100kg
suvorexant

US $48.00-40.00/KG2023-05-04
- CAS:
- 1030377-33-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20tons