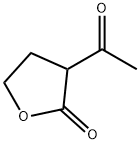
Advances in 2-Acetylbutyrolactone: Chemistry, Transformations, and Applications
Introduction
2-Acetylbutyrolactone is a valuable intermediate for the preparation of active ingredients for drugs, pesticides and various heterocyclic compounds. It is also a useful reagent for the synthesis of many N-heterocycles. The condensation reactions (i.e. Schiff base formation reactions) of 2-Acetylbutyrolactone with aniline or 4-fluoro aniline to form 2-(1-phenylimino)ethyl-γ-butyrolactone and 3-[1-(4fluoro-phenylamino)ethylidene]dihydro-2(3H)-furanone. [1]

Method for Preparing 2-acetylbutyrolactone
The present invention relates to a method for the preparation of 2-acetylbutyrolactone from γ-butyrolactone and an acetic acid ester by reacting these two compounds in the presence of a strongly basic condensation agent, followed by protonation of the initially formed enolate, wherein the reactants are fed continuously into a reaction zone in which the condensation reaction takes place and from which the reaction mixture is drawn off, either batchwise or continuously. The reactants are used in a ratio of from 1.0 to 6.0 parts by mols of acetic acid ester and from 0.9 to 1.6 parts by mols of the strongly basic condensation agent per part by mols of γ-butyrolactone. After the reaction mixture is withdrawn from the reaction zone, it is protonated to provide the final product. The method according to the present invention provides a number of surprising advantages. The 2-acetylbutyrolactone is obtained, after a single distillation, in >99% purity and in excellent yields of more than 90%, based on γ-butyrolactone used. Thus, the γ-butyrolactone is reacted with a high degree of selectivity. Side reactions and/or secondary reactions, which reduce the selectivity and the yield of 2-acetylbutyrolactone, are largely suppressed. Thus hydroxy- or alkoxybutyric acid derivatives, for example, which not only reduce the yield but cannot easily be separated by distillation from 2-acetylbutyrolactone, are produced in considerably smaller amounts than with the prior art methods. [2]
![Article illustration]()
![Article illustration]() Stereoselective Reduction of 2-acetylbutyrolactone
Stereoselective Reduction of 2-acetylbutyrolactone
The product of the reduction of 2-acetylbutyrolactone—α’-1’-hydroxyethyl-γ-butyrolactone possesses two stereogenic centres and may be classified as a β-hydroxyester. This compound is a structural analog of γ-butyrolactone (GLB), which exerts several inhibitory action over the central nervous system (CNS) through the interaction with neuronal high-affinity receptors. Isomers of α’-1’-hydroxyethyl-γ-butyrolactone could be potential ligands of the same receptors. C. viswanathi AM120 transformed α-acetylbutyrolactone preferentially to (3S,1’S)-α’-1’-hydroxyethyl-γ-butyrolactone. With reference to Y. lipolytica strains, the study found that substrate was converted highly enantioselectively to (3R,1’R)-α’-1’-hydroxyethyl-γ-butyrolactone alone by all used strains. The initiation of biotransformation with resting cell cultures decreased the enantioselectivity of the reduction. The modification of the reaction medium by addition of small amounts of organic solvents resulted in an increase in the substrate’s reactivity but a decrease in the enantioselectivity of the process. Consequently, the addition of DES to the cultures of C. viswanathi AM120 at 10% and 25% decreased the time of transformation, and the stereoselectivity of the process was enhanced. [3]
Application of 2-acetylbutyrolactone to Spectrofluorimetry
2-Acetylbutyrolactone has been characterized for use as a fluorogenic reagent for the spectrofluorimetric determination of primary amines. In the current work, the inherent fluorescence characteristics of 2-acetylbutyrolactone (a furanone derivative) and of Schiff bases derived from 2-acetylbutyrolactone reaction with some amines of type RNH2 or ArNH2 were studied. Based on these results, 2-acetylbutyrolactone is proposed as a fluorogenic agent for primary amines. Concerning the 2-acetylbutyrolactone Schiff base reaction, first, the reaction can be carried out in both aqueous and organic media. Second, it reacts efficiently with primary amines of both aryl and aliphatic types. Third, the Schiff bases exhibit fluorescent properties that allow fluorimetric analysis down to the ng (s) level. 2-acetylbutyrolactone can therefore be used as well as the other, well known, fluorimetric reagents specified for primary amines like fluorescamine, o-phthalaldehyde, dansyl chloride and naphthalenedialdehyde. The reaction of such reagents with primary amines occurs in aqueous solutions with suitable pH adjustment. Fluorescamine keeps the advantage of very fast reaction, which makes it suitable for post-colum derivatization, the matter which is not an option for the 2-acetylbutyrolactone reaction. [4]
References:
[1] WAMHOFF, H., & KORTE, F. (1972). The Synthesis of Heterocyclic Compounds Starting from Lactones, Lactams, and Thiollactones1. Synthesis, 1972(04), 151-175.
[2] https://patents.google.com/patent/US5789603A/en
[3] M?czka, W., Wińska, K., Grabarczyk, M., & ?arowska, B. (2018). Yeast-Mediated Stereoselective Reduction of α-Acetylbutyrolactone. Applied Sciences, 8(8), 1334. https://doi.org/10.3390/app8081334
[4] Sabry, S. M. (2006). Application of 2-acetylbutyrolactone to spectrofluorimetry: Fluorescence properties of Schiff bases derived from 2-acetylbutyrolactone and spectrofluorimetric determination of primary amine-containing compounds. Journal of pharmaceutical and biomedical analysis, 40(5), 1057-1067.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Acetylbutyrolactone manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 517-23-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $0.00/kg2025-04-21
- CAS:
- 517-23-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000mt