A naturally antibiotic:Mupirocin
DESCRIPTION
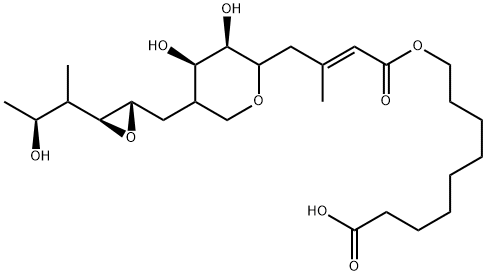
Mupirocin, formerly called pseudomonic acid A, is a naturally occurring antibiotic that was originally isolated as a fermentation product from Pseudomonas fluorescens. It binds reversibly to the bacterial isoleucyl transfer-RNA synthetase and thereby inhibits bacterial protein synthesis. This mechanism of action is unique among antibiotics and thus circumvents the development of antibiotic cross-resistance. Mupirocin is exclusively used topically as it is rapidly inactivated in plasma. It is used for the treatment of superficial infections, particularly due to staphylococci and streptococci, and to eliminate nasal carriage of Staphylococcus aureus. It is available as cream, spray, or ointment. Trade names for mupirocin include Bactroban, Centany, Mupirocin calcium, Pseudomonic acid A, MRC and Turixin.
MECHANISM OF DRUG ACTION
Antibacterial activity of P. fluorescens was first recorded in 1887 by Baader and Garre, but it was not until 1971 that Fuller et al. isolated mupirocin, the major metabolite which accounted for most of this activity. Mupirocin contains the biogenetically unique 9-hydroxy-nonanoic acid moiety, a molecule which has some resemblance with isoleucyl and binds reversibly to the class I isoleucyl-tRNA synthetase from several eubacteria. The synthetase is responsible for the formation of isoleucyl-tRNA by means of the aminoacylation of isoleucine to the cognate tRNA. Isoleucyl-tRNA synthesis occurs in two steps. First, the intermediate isoleucyl-adenylate (isoleucyl-AMP) is generated from isoleucine and ATP. The second step involves the transfer of this intermediate to the 3u-terminus of the corresponding tRNA. This isoleucyl-tRNA complex subsequently delivers the isoleucines to ribosomes during protein synthesis. Mupirocin acts as an analog of isoleucyl-AMP by binding to the catalytic cleft of the tRNA synthetase, called the Rossman fold domain. Competitive inhibition of this enzyme arrests protein synthesis.
MODE OF DRUG ADMINISTRATION
Mupirocin is exclusively used topically as cream or ointment. It is well absorbed after oral and parenteral administration, but its serum concentrations are short-lived due to extensive degradation to monic acid, an antibacterially inactive metabolite. For treatment of skin infections such as impetigo, mupirocin is available as 2% ointment in polyethylene glycol base. This base is, however, irritant to mucous membranes, open wounds, or burns. For treatment of these areas another formulation of the agent, 2% calcium mupirocin in a white soft-paraffin base, has been developed. This is suitable for the application to the nasal mucosa. The dose is similar for all patients, regardless of age. The formulation is applied locally to lesions two or three times daily for up to 10 days, depending on the response.
PHARMACOKINETICS
Mupirocin is poorly absorbed through the intact human skin (less than 0.24%). If it enters the circulation, e.g. through wounds, 97% binds to serum proteins (plasma binding). Mupirocin is rapidly metabolized in the liver, with a half-life of 20–40 minutes, and converted to the microbiologically inactive and nontoxic metabolite monic acid. Excretion is mainly by the kidney (90%). The rapid inactivation in plasma and high plasma binding make mupirocin unsuitable for systemic use. The relationship between susceptibility and response is described earlier under 2b. Emerging resistance and cross-resistance.
TOXICITY
Mupirocin is well tolerated. Less than 2% of users of topical treatment report burning, stinging, pain, or headache; 1% complain about itching. Less than 1% report rash, erythema, dry skin, tenderness, swelling, increased exudate, contact dermatitis, or nausea. Mupirocin formulations containing polyethylene glycol should be used with caution in patients who suffer from moderate or severe renal Mupirocin 981 impairment. The excretion of polyethylene glycol may be impaired and could lead to nephrotoxicity and severe metabolic disturbances.
CLINICAL USES
Topical antibiotics have a number of advantages over systemic antibiotics. Topical use generally circumvents systemic allergic reactions and side-effects and has the advantage that high antibiotic concentrations can be achieved at the site of infection. Mupirocin is indicated for the topical treatment of localized primary and secondary bacterial skin infections caused by S. aureus, S. pyogenes, and other susceptible organisms. It is furthermore used to prevent skin infections after minor abrasions, wounds, or insect bites and to eradicate S. aureus carriage, including MRSA. Resistance to mupirocin appears to be increasing and can rapidly spread in a setting of endemic MRSA colonization.
Related articles And Qustion
Lastest Price from Mupirocin manufacturers

US $1.00-100.00/KG2025-09-04
- CAS:
- 12650-69-0
- Min. Order:
- 1KG
- Purity:
- 95%
- Supply Ability:
- 300KG

US $5.00-0.50/KG2025-06-11
- CAS:
- 12650-69-0
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg