Perindoprilerbumine: A Comprehensive Overview
What is Perindopril?
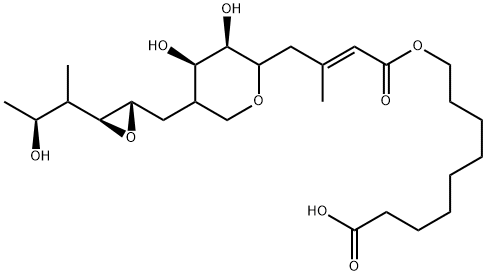
Perindopril (2S,3aS,7aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxopen-tan-2-yl]amino]propanoyl] 2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic acid) belongs to the class of long acting angiotensin-converting enzyme (ACE) inhibitors, which are effective in the treatment and prevention of several medical conditions (such as reducing blood pressure, reversing abnormalities of vascular structure and function in patients with essential hypertension, congestive heart failure, post-myocardial infarction, diabetic nephropathy). Besides antihypertensive effect, ACE inhibitors exhibit also vasculoprotective and antithrombotic activities that play a favorable role in terms of cardiovascular morbidity.
Perindopril is an acid-ester prodrug, which is deesterified in the liver by esterases. The active form of perindopril-perindoprilat is diacid. Pascard et al. determined configuration and preferential solid-state conformation of perindoprilat, and more recently theoretical calculations of molecular structure and stability of the arginine and erbumine salts of perindopril were also carried out.
The physicochemical and pharmacokinetic properties of ACE inhibitors and their active metabolites were also theoretically examined. Perindopril is observed to be chemically unstable and undergoes degradation in dosage forms to form diketopiperazines and diacids. Therefore in the therapeutical praxis perindopril is orally administered in the form of tablets containing physiologically acceptable salts (1:1) with Perindoprilerbumine (tert-butylamine) and L-arginine (perindopril erbumine and perindopril L-arginine).

Figure 1 Characteristics of Perindoprilerbumine
Crystallization
Crystallization of perindoprilerbumine was not an easy task. After several weeks of growth, a few rather big crystals were formed by slow evaporation of water or methanol-ethanol solution at room temperature. The crystals grown from an aqueous solution have the shape of elongated prisms, whereas those crystallized from the methanol–ethanol solution are the shape of needles.
Two monocrystals representative for the two observed habits were chosen for the X-ray experiment. The selected crystals turned out to be polymorphic forms of the perindopril erbumine salt. The prismatic one had triclinic P1 symmetry, while the needle-like one had monoclinic P21 symmetry.
Theoretical Calculations
The structure of the perindoprilerbumine salt undergoing restricted thermal motion in a crystal field and measured by single-crystal X-ray diffraction is discussed. The structure of the isolated perindopril erbumine salt is the definitive structure from the point of view of the ab initio theoretical chemist.
The initial conformations for use in the ab initio calculations were previously analyzed experimental structural data obtained from X-ray crystallography. The HF and B3LYP DFT bond lengths and bond angles of salt studied fit one another to within about 0.02 Å for bond lengths and about 3° for bond angles. Larger differences (about 5–9°) were observed for dihedral angles. The geometry of the hydrogen bonding moiety COO-.....H3 N+ is described by both ab initio methods by the same way. The initial calculations of the perindoprilerbumine were carried out for the complexes involving charged (ionic) hydrogen bonds. In these complexes the proton is transferred from the perindopril to the erbumine. According to our calculations both, HF and DFT methods suggest that the most stable structure is stabilized via neutral hydrogen bond of the COOH....N type. It means that in the isolated state perindoprilerbumine will exist as neutral hydrogen bonded complex.1
References:
[1] M. REMKO . Crystal and molecular structure of perindopril erbumine salt[J]. Journal of Molecular Structure, 2011, 997 1: 1-130. DOI:10.1016/j.molstruc.2011.05.005.Related articles And Qustion
See also
Lastest Price from Mupirocin manufacturers

US $1.00-100.00/KG2025-09-04
- CAS:
- 12650-69-0
- Min. Order:
- 1KG
- Purity:
- 95%
- Supply Ability:
- 300KG

US $5.00-0.50/KG2025-06-11
- CAS:
- 12650-69-0
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg