A antiperspirant product: Aluminum chlorohydrate
General description
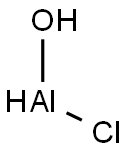
Aluminum chlorohydrate is a water soluble aluminum complex which contains several oligomeric polycations of aluminum. In acidic or neutral conditions, aluminum monomer Al3+ do form complexes with H2O molecules and OH− ions leading to the aluminum monomer of general formula. Polycondensation of aluinum monomers leads to polycationic oligomers with degrees of polymerization up to Al13or Al30, that were described as a layut of octahedral structures within the “cage-like” Keggin model. The interactions of those aluminum polycationic oligomers with bovine serum albumin among other proteins were investigated by Deschaumes et al.
Figure1 the chemical structure of Aluminum chlorohydrate
Application and Pharmacology
Antiperspirant activity is of primary interest in the cosmetic industry as antiperspirants and deodorants are of common use worldwide. Deodorants attempt to control body odor by acting on the bacterial flora of the underarm area, whereas antiperspirants act on reducing the sweat flow by obstructing sweat ducts. Aluminum chlorohydrates (ACH) are the active ingredients in most antiperspirant formulations. Their synthesis is based on the neutralization of aluminum chloride by metallic aluminum, leading to highly concentrated aluminum polycation solutions. Some antiper spirants are formulated from similar aluminum salts which may also contain zirconium Analysis and characterization of aluminum chlorohydrate oligocations by capillary electrophoresis. Aluminum chlorohydrates (ACH) are the active ingredients used in most antiperspirant products. ACH is a water soluble aluminum complex which contains several oligomeric polycations of aluminum with degrees of polymerization up to Al13or Al30. The characterization and quantification of ACH oligo-cations remain a challenging issue of primary interest for developing structure/antiperspirant activity correlations, and for controlling the ACH ingredients. In this work, highly repeatable capillary electrophoresis (CE) separation of Al3+, Al13and Al30oligomers contained in ACH samples was obtained at pH 4.8, owing to a careful choice of the background electrolyte counter-ion and chromophore, capillary I.D. and capillary coating. This is the first reported separation of Al13and Al30oligomers in conditions that are compatible with the aluminum speciation in ACH solution or in conditions of antiperspirant application/formulation. Al13and Al30 effective charge numbers were also determined from the sensitivity of detection in indirect UV detection mode. The relative mass proportion of Al13 compared to Al13+ Al30 could be determined in different aluminum chlorohydrate samples. Due to its simplicity, repeatability/reproducibility, minimal sample preparation and mild analytical conditions, CE appears to be a promising analytical separation technique for the characterization of ACH materials and for the study of structure/antiperspirant activity correlations[1].
Safety
The terms deodorants and antiperspirants very frequently used interchangeably despite the fact that they employ completely different active substances and mechanisms of action. Antiperspirants are necessarily deodorants due to the lack of substrate to decompose. They nevertheless represent a group of very specific substances that create particular problems due to the presence of aluminium chlorohydrate, or ACH, (Al2(OH)5Cl, 2H2O), aluminium sesquichlorohydrate and aluminium-zirconium complex, which, after hydrolysis, causes intense acidification of the skin, hence the importance of inclusion of emollients and pH regulators in formulations. Moreover , systemic aluminium is thought to be genotoxic and to promote breast cancer , and it is thus at the centre of numerous scientific controversies. Nevertheless, its potential toxicity following topical application is related to its ability to penetrate skin, which is as yet poorly understood but considered very low , a fact that may provide some degree of reassurance regarding its use in cosmetic products. Its role in Alzheimer’s disease has not been proven. On the other hand, zirconium salts are considered toxic and are partly regulated in Europe. The problems associated with deodorants are those arising from the presence of antiseptics (triclosan, usnic acid) capable of inducing bacterial resistance, but more particularly , the presence of axillary dermatitis due to the alergenic potential of the fragrances and essential oils used (e.g. isoeugenol, citronellal, lyral, cinnamic aldehyde, etc.)[2]
Reference
1.Ouadah N., Moire C. & Kuntz J. et al., "Analysis and characterization of aluminum chlorohydrate oligocations by capillary electrophoresis," Journal of Chromatography A, Vol.1492(2017), pp.144-150.
2.Martini M. C., "Déodorants et antitranspirants," Annales de Dermatologie et de Vénéréologie, Vol.147, No.5(2020), pp.387-395.
You may like
Related articles And Qustion
See also
Lastest Price from Aluminum chlorohydrate manufacturers
US $0.00-0.00/kg2025-11-28
- CAS:
- 1327-41-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $6.00/kg2025-04-21
- CAS:
- 1327-41-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month