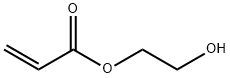
Molecular structure data of 2-hydroxyethyl acrylate
Background
Plastics generally consist of large molecules called polymers as well as additives that are used to modify the properties of the material. Polymers are formed by the stepwise reaction between monomers by which larger molecules are formed. In this polymerization process, primarily dimers, trimers, and higher molecular weight (MW) molecules (i.e., oligomers) are formed until finally the polymer is completed. 2-Hydroxyethyl acrylate is a important monomer. If only one type of monomer is involved in forming the polymer, it is called a homopolymer, e.g., polyethylene. If two or more different monomers are used, it is called a copolymer, e.g., propylene ethylene copolymer. This type of polymerization is usually controlled by the addition of a small amount of catalyst or initiator. Another terminology is used for the production of, for example, 2-hydroxyethyl acrylate plastics. In the formation of 2-hydroxyethyl acrylate plastics, several auxiliary compounds are added to modify the final product. Examples of groups or classes of additives are plasticizers, fillers, antioxidants, UV-stabilizers, antiozonants, antistatic agents, biostabilizers, heat stabilizers, blowing agents, flame retardants, dyes, fragrances, etc.
Picture 1 2-Hydroxyethyl acrylate
Photocuring
It is common to define photocuring as a process of rapid conversion of specially formulated, usually liquid solventless compositions into solid films by irradiation with ultraviolet or visible light. This is used to form films of varnishes, paints, adhesives, and coatings for paper, plastic, wood, metal surfaces, optical fibers and compact discs. 2-hydroxyethyl acrylate is used in the photocuring, like preparation of printing plates, photoresists, in graphic arts, in microelectronics, and in stereo- and microlithography. In addition 2-hydroxyethyl acrylate is also utilized in dental restorative fillers, fiber-optic treatment, aspherical lenses for CD applications, and in preparations of some contact lenses where acrylate and methacrylate compositions are efficiently crosslinked. Typical photocuring reactions of 2-hydroxyethyl acrylate are very rapid, lasting from sub-second to minute time scales.
The serum replacement technique
The serum replacement technique has been used to “clean” an industrial ethyl aerylate-methyl methacrylate copolymer latex prepared in the presence of 2-hydroxyethyl acrylate monomer. The “cleaning” method allowed the separation of the cleaned latex particles from the serum in a form suitable for further analysis. Both the cleaned latex particles and the serum were analyzed by several methods. Serum replacement recovered about 90% of an anionic surfactant and almost all of the nonionic surfactant used in the preparation of the latex. 17.6% of the initiators used in the preparation of the latex were found to be chemically-bound to the surface of the particles. The 2-hydroxyethyl acrylate monomer found on the surface of the particles and in the aqueous phase amounted to 29.85% and 28.61%, respectively, of the total amount of acrylic acid used in the preparation of the latex. The rest of the 2-hydroxyethyl acrylate was assumed to be buried inside the latex particles.
Grafting of 2-hydroxyethyl acrylate monomers
Grafting of 2-hydroxyethyl acrylate monomers onto cellulose is an important tool for the modification of cellulose. Depending on the monomer grafted onto cellulose, it gains new properties. The grafting can be performed in heterogeneous or homogeneous medium. In the grafting performed in heterogeneous medium, the reaction is carried out in aqueous medium using a suitable initiator. As initiator, the radiation or chemical initiators such as ceric ammonium nitrate (CAN), various persulfates, azobisisobutyronitrile (AIBN), and Fenton reagent (Fe(II)–H2O2) are mostly used. In case of CAN initiator, the grafting should be performed in acidic medium in order to prevent its hydrolysis. In the homogeneous grafting reactions, either a water-soluble cellulose derivative is used in the grafting or cellulose is dissolved in a suitable solvent, and then the grafting is performed. Higher number of grafts per cellulose chain is obtained in homogeneous grafting than those in heterogeneous medium.
The previous chapter has dealt with the general principles which underlie the production of synthetic latices by emulsion polymerization. This chapter reviews the individual types of synthetic latex. The concern is exclusively with aqueous synthetic latices. Non-aqueous synthetic latices are also used industrially; they are produced by emulsion/dispersion polymerization in non-aqueous media. However, aqueous synthetic latices far exceed non-aqueous synthetic latices as regards multiplicity and industrial importance. The main concern in this chapter is, of course, with synthetic latices which have attained industrial importance to a greater or lesser extent. However, brief reference is also made to certain other interesting types which so far have either been of very limited specialist applicational interest only, or remained of essentially academic interest. Almost all the synthetic latices of industrial importance are produced by free-radical addition polymerizations of olefinically-unsaturated monomers in aqueous dispersion media.
Reference
1 Bert Björkner, Malin Frick-Engfeldt, Ann Pontén, Erik Zimerson in Contact Dermatitis (2011)
2 A. Ravve in Light-Associated Reactions of Synthetic Polymers (2006)
3 M. S. El-Aasser, S. M. Ahmed, G. W. Poehlein, J. W. Vanderhoff… in Polymer Colloids II (1980)
4 Gülten Gürdağ, Shokat Sarmad in Polysaccharide Based Graft Copolymers (2013)
5 D. C. Blackley in Polymer Latices (1997)
Related articles And Qustion
See also
Lastest Price from 2-Hydroxyethyl acrylate manufacturers

US $2.00-6.00/KG2025-07-28
- CAS:
- 818-61-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-ton

US $1.00/kg2025-04-21
- CAS:
- 818-61-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt