4-Methoxyphenol: Applications in Electrochemical Detection and its Carcinogenicity
General Description
4-Methoxyphenol can be utilized as a polymerization inhibitor, UV inhibitor, dye intermediate, and in antioxidant synthesis for oils and cosmetics. In the field of electrochemical detection, 4-methoxyphenol acts as a redox reporter for real-time profiling of H2O2 in living cells, aiding in understanding cellular redox processes. However, caution is advised due to its potential toxicity and flammability. Additionally, studies have shown that 4-methoxyphenol exhibits forestomach carcinogenicity in rats, highlighting the importance of handling this compound with care.

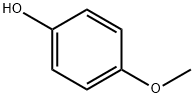
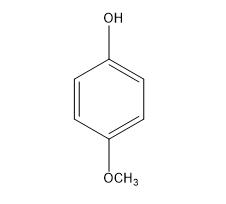
Figure 1. 4-Methoxyphenol
Applications in Electrochemical Detection
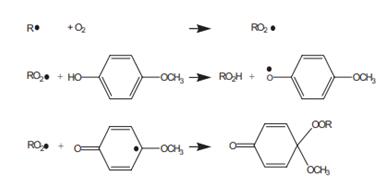
Hydrogen peroxide (H2O2) plays a persuasive role in the human cell physiology. Developing an efficient assay platform and a highly sensitive tracking and quantification of H2O2 in a physiological system is crucial to understand the neoplastic changes and/or redox homeostasis of cells. In this study, a novel turn-on latent electrochemical redox probe coupled with electrocatalytic signal amplification strategy is proposed. A custom-made readily available turn-on latent electrochemical probe 4-methoxyphenylboronic acid pinacol ester (4-MPBP) have been designed for the selective detection of endogenous H2O2 in live cells. The electrochemical probe composed of a latent electrochemical reporter (4-methoxy phenol, 4-MP) bearing a recognition unit (boronic acid pinacol ester) for H2O2 sensing. The selective analyte-triggered chemical transformation releases free electrochemical reporter 4-MP. The amount of H2O2 was evaluated electrochemically at glassy carbon electrode (GCE) with a broad detection range of 0.5 μM−1 mM. An amplified signal response of released 4-MP to build a highly sensitive assay tool has been achieved via replacing the GCE transducer electrode with polydopamine@carbonnanotube−molebtinumdisulfie hybrid modified GCE as it delivered an exceptional dynamic detection range of 0.01−100 μM. The innovative blend of electrochemical molecular probe strategy, with electrocatalytic signal amplification technique has delivered outstanding assay performance at trace level sensing of H2O2. Next, we set up a platform for real-time in vivo monitoring of the endogenously produced H2O2 in Caco-2 and MCF-7 cells through spermine−polyamine analogue and phorbol 12-myristate 13-acetate induction in SSAT/PAO gene and protein kinase C, respectively. As expected, the 4-MPBP latent probe coupled with electrocatalytic signal amplification strategy delivered outstanding performance for in situ H2O2 release and tracking over time.1
Carcinogenicity
The carcinogenic potentials of 4-methoxyphenol (4-MP) and 4-methylcatechol (4-MC), phenolic compounds which are structurally similar to the known forestomach carcinogen BHA and the glandular stomach carcinogen catechol respectively, and cause considerably enhanced cell proliferation and cytotoxicities in rat forestomach and/or glandular stomach epithelium, were examined in male and female F344 rats. Groups of 30 male and female animals were administered diets containing 2% 4-MP or 2% 4-MC for 104 weeks. Histopathological findings in the 4-MP case included atypical hyperplasias (male, 67%, female, 37%), papillomas (50%, 23%) and squamous-cell carcinomas (77%, 20%) in the forestomach. 4-MC induced forestomach papillomas (70%, 93%) and squamous-cell carcinomas (53%, 37%), also glandular stomach submucosal hyperplasias (90%, 93%), adenomas (100%, 100%) and adenocarcinomas (57%, 47%), with ulceration or erosion. The degree of differentiation of the squamous-cell carcinomas induced by 4-MP was less than with 4-MC. The present study demonstrated unequivocal forestomach carcinogenicity for 4-MP and forestomach and glandular stomach carcinogenicity for 4-MC, with cytotoxicity and cell proliferation both appearing as important factors for these non-genotoxic carcinogens.2
References:
[1] KESAVAN MANIBALAN. Latent Redox Reporter of 4-Methoxyphenol as Electrochemical Signal Proxy for Real-Time Profiling of Endogenous H2O2 in Living Cells[J]. ACS Sensors, 2019, 4 9: 2213-2554. DOI:10.1021/acssensors.9b01049.[2] EMIKO ASAKAWA. Carcinogenicity of 4-methoxyphenol and 4-methylcatechol in F344 rats[J]. International Journal of Cancer, 1994, 56 1: fmi, 1-162. DOI:10.1002/ijc.2910560126.
Related articles And Qustion
See also
Lastest Price from 4-Methoxyphenol manufacturers

US $0.00/kg2025-08-13
- CAS:
- 150-76-5
- Min. Order:
- 25kg
- Purity:
- 99.94%
- Supply Ability:
- 1000 tons

US $0.00/kg2025-08-08
- CAS:
- 150-76-5
- Min. Order:
- 25kg
- Purity:
- 99.94%
- Supply Ability:
- 1800