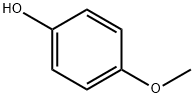
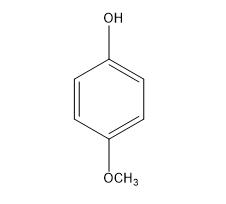
Inhibiting action of 4-Methoxyphenol for Acrylic Monomers
4-Methoxyphenol (MeHQ) is widely used in chemistry field, such as the stabilizer to inhibit peroxide formation in ethers, chlorinated hydrocarbons and ethyl cellulose, and an intermediate to manufacture other stabilizers, dyes, pharmaceuticals and plasticizers [1]. The most important role of MeHQ is acting as the inhibitor in radical polymerization monomers, such as acrylic monomers.
An inhibitor of acrylic monomers ensures the safe and efficient operation of manufacturing plants and the safe storage of acrylic monomers. If without inhibitor in monomers, the strongly exothermic polymerization constitutes a significant safety risk during production, workup, as well as in storage and transportation. On the one hand, the released heat can cause deflagration and explosions. On the other hand, the polymers can lead to blockages and breakdowns in parts of the production plant, leading to losses in production and higher maintenance costs [2]. Therefore, it is necessary to add polymerization inhibitor in the monomers.
Let see what happen as inhibitor present in the monomers. Take acrylic acids (AA) for example, MeHQ works together with the oxygen, which dissolve in monomer under normal conditions and air coverage. Three main steps explain the inhibiting action.
(1) Oxygen reacts with the primary (carbon) radicals produced by the initiator and forms peroxy radicals. (2) Monomer adds to the peroxy radicals in a much slower rate to form a random copolymer. (3) The peroxy radicals easily terminate each other, produce dead copolymer, and release an oxygen molecule. The steps show that, MeHQ, the inhibitor does not react directly with the primary radicals (R•) of the monomer, and the primary radicals react with O2 to form peroxide radicals (RO2•), which is trapped up by the reaction with MeHQ (see Scheme 1) [3]. That is to say, MEHQ is more likely to react with peroxy radicals and to form more stable radicals that may further terminate peroxy radicals. Thus, MEHQ prevents the formation of long oxygen−monomer copolymer chains and, therefore, reduces the consumption rate of oxygen and enhances the inhibition of oxygen [4].
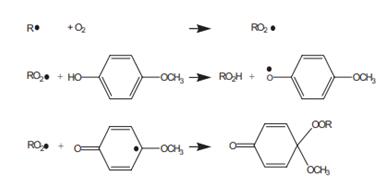
Scheme 1 Reaction mechanism
Based on the consumption of the oxygen and MeHQ, conclusions can be drawn to the stability of the monomers.
Reference
[1] https://pubchem.ncbi.nlm.nih.gov/compound/4-Methoxyphenol
[2] Jaroslav Mosnáček etc. Efficient Polymerization Inhibition Systems for Acrylic Acid Distillation: New Liquid-Phase Inhibitors, nd. Eng. Chem. Res.201251103910-3915
[3] Holger Becker1 Herbert Vogel1, The Role of Hydroquinone Monomethyl Ether in the Stabilization of Acrylic Acid, Chem. Eng. Technol. 2006, 29, No. 10, 1227–1231
[4] Rujun Li, F. Joseph Schork, Modeling of the Inhibition Mechanism of Acrylic Acid Polymerization, Ind. Eng. Chem. Res.20064593001-3008.
You may like
Related articles And Qustion
See also
Lastest Price from 4-Methoxyphenol manufacturers

US $0.00-0.00/KG2025-12-16
- CAS:
- 150-76-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $0.00/kg2025-08-13
- CAS:
- 150-76-5
- Min. Order:
- 25kg
- Purity:
- 99.94%
- Supply Ability:
- 1000 tons