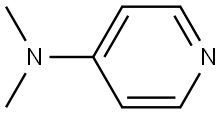
4-Dimethylaminopyridine: An Effective Acyl Transfer Agent
General Description
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine. Its basicity makes it a useful nucleophilic catalyst for a variety of reactions. It is used as an acylation catalyst in the synthesis of agrochemicals, pharmaceuticals and polymers and as a coupling agent for the synthesis of peptide. It is used as an intermediate in the pharmaceutical industry for the synthesis drugs including anti-HIV treatment, analgesics and a chemotherapy drug.

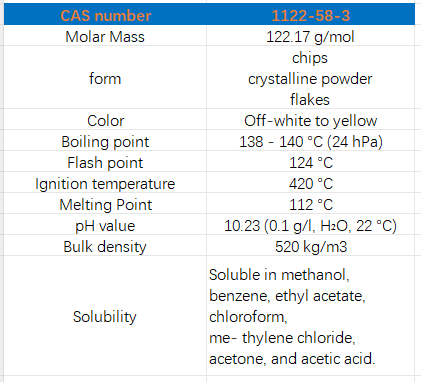
Figure 1. Properties of 4-Dimethylaminopyridine
Preparation of 4-Dimethylaminopyridine
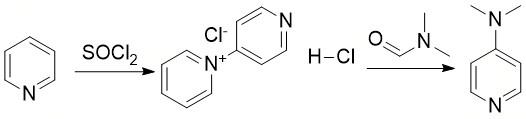
Figure 2. Preparation of 4-Dimethylaminopyridine
The process comprises initially charging ethyl acetate and pyridine, one by one, under agitation, and maintaining the pot temperature at 25±5° C. Thionyl chloride is then charged gradually while maintaining the temperature below 35° C. After the addition, the reaction mass is refluxed for 4 hours at 77-80 °C. After refluxing, the ethyl acetate and unreacted thionyl chloride are distilled off at atmospheric pressure. Finally, mild vacuum (400 to 600 mm Hg) is applied to ensure maximum recovery of ethyl acetate and thionyl chloride. Left over reaction mass is cooled to 40 °C. Then anhydrous ethyl alcohol is added slowly while maintaining the temperature below 60 °C. The resulting reaction mass is stirred well, then cooled up to 10° C. and then filtered, washed with anhydrous ethyl alcohol and dried under vacuum to get the desired salt N-[4-pyridyl] pyridinium chloride hydrochloride.1
The obtained salt N-[4-pyridyl] pyridinium chloride hydrochloride is aminated with N,N-dimethyl formamide. The process comprises charging N-[4-pyridyl] pyridinium chloride hydrochloride salt and N,N-dimethyl formamide one by one. The reaction mass is slowly heated to raise the temperature 50 to 60 °C. The agitator is started, and the reaction mass is further heated slowly to a reflux temperature of 140 to 150 °C. Reflux is continued for 2 hours at 150 to 155 °C. After 2 hours of refluxing, the reaction mass is distilled first atmospherically and then under vacuum to recover pyridine to the maximum possible extent. The reaction mass is cooled up to 40 °C. and then hydrolysed by adding 10% caustic lye solution, while maintaining the pH at 11 to 12. The neutralised reaction mass is further cooled to 20 °C, and the precipitated inorganic cake is filtered off. The mother liquor is extracted with benzene in multiple steps to ensure almost complete extraction. The extracted reaction mass is distilled atmospherically and then under vacuum to recover benzene. The crude 4-dimethylaminopyridine left in the pot is distilled under high vacuum employing a fractionating column to get white to off-white crystals of 4-dimethylaminopyridine in high yield and purity.1
Common Uses of 4-Dimethylaminopyridine
Nucleophilic catalyst for Boc protections
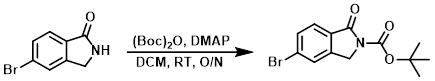
Figure 3. The Application of 4-Dimethylaminopyridine in Boc Protections
A mixture of 5-bromoisoindolin-1-one (40 mg, 0.19 mmol), di-tert-butyl dicarbonate (41 mg, 0.19 mmol), 4-dimethylaminopyridine (2.3 mg, 0.02 mmol) in dichloromethane (3 mL) was stirred at room temperature overnight. When TLC (ethyl acetate) indicated the starting material was consumed, the reaction mixture was washed with water and brine, dried over anhydrous sodium sulfate, and evaporated in vacuo to give tert-butyl 5-bromo-1-oxoisoindoline-2-carboxylate (30 mg, 51% yield) as a white solid.2
Reagent for amide couplings (EDCI + DMAP)

Figure 4. The Application of 4-Dimethylaminopyridine in Amide Couplings
To a stirred solution of 2-bromoaniline (150 g, 872 mmol, 1 eq.) and 4-dimethylaminopyridine (138.5 g, 1133 mmol, 1.3 eq.) in CH2CI2 (2500 ml) was added N-tert-butoxycarbonyl )azctidinc-3-carboxylic acid (872 mmoles, 1 eq) in one portion followed by the addition of EDCI (217 g, 1133 mmol, 1.3 eq.) in one portion at room temperature. The resulting mixture was stirred at room temperature overnight. It was then successively washed with 10% citric acid aqueous solution, water, saturated Na2CO3 aqueous solution, and brine, and dried over Na2SO4. After filtration, the solvent was removed under vacuum to give tert-butyl 3-((2-bromophenyl)carbamoyl) azetidine-1-carboxylate (328 g, 85% yield).3
Reagent for the conversion of carboxylic acids to esters (Steglich Esterification)
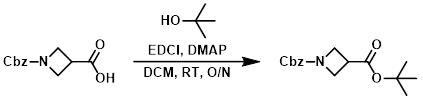
Figure 5. The Application of 4-Dimethylaminopyridine in Steglich Esterification
To a solution of l-(benzyloxycarbonyl)azetidine-3-carboxylic acid (200 g, 0.851 mol) in dichloromethane (6.0 L) at 0 °C was added /-butanol (158 g, 2.13 mol), 4-dimethylaminopyridine (52.0 g, 0.425 mol), and EDCI (163 g, 0.853 mol). The reaction mixture was stirred at room temperature overnight. Next, the reaction mixture was concentrated and the residue was dissolved in ethyl acetate. The organic layer washed with 10% aqueous citric acid, 10 % aqueous sodium bicarbonate solution, and brine. Drying over anhydrous sodium sulfate and concentration under reduced pressure afforded 1-benzyl-3-tert butyl-azetidine-1,3-dicarboxylate (200 g, 81% yield) as a colorless oil.4
Nucleophilic catalyst for TBS protections (silylation)
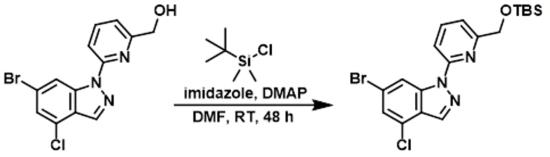
Figure 6. The Application of 4-Dimethylaminopyridine in TBS protections
A mixture of [6-(6-Bromo-4-chloro-indazol-1-yl)-pyridin-2-yl]-methanol (0.750 g, 2.22 mmol), 1H-Imidazole (0.302 g, 4.43 mmol), tert-Butyldimethylsilyl chloride (0.401 g, 2.66 mmol) and 4-Dimethylaminopyridine (0.01 g, 0.08 mmol) in N,N-Dimethylformamide (6.86 mL, 88.6 mmol) was stirred for 48h at rt. The reaction was diluted (DCM), washed (NaHCO3), and evaporated. The residue was purified (Si02 40g heptane → EtOAc) to afford 600 mg of clear solid.5
Nucleophilic catalyst for the conversion of alcohols to triflates

Figure 7. The Application of 4-Dimethylaminopyridine in Conversion of Alcohols to Triflates
To a solution of 4- bromo-2-nitrophenol (5.0 g, 22.9 mmol) in dichloromethane (100 mL), triethylamine (2.8 g, 27.5 mmol), 4-dimethylaminopyridine (280 mg, 2.3 mmol) and trifluoromethanesulfonic anhydride (7.8 g, 27.5 mmol) were added dropwise at 0 °C. The resulting mixture was stirred at room temperature for 1 h. After completion of the reaction, the reaction mixture was partitioned between dichloromethane and water. The organic phase was washed with saturated sodium bicarbonate solution followed by brine solution. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography to afford the desire product (7.2 g, 90%) as a yellow oily liquid.6
Safety
4-Dimethylaminopyridine is considered to be highly toxic. The fact that DMAP is readily absorbed through the skin makes it even more dangerous.
References
1. US6939972B2
2. WO2010027500
3. WO2015158653
4. WO2011017578
5. WO2016011390
6. WO2014149164
Related articles And Qustion
See also
Lastest Price from 4-Dimethylaminopyridine manufacturers

US $50.00-10.00/kg2025-09-02
- CAS:
- 1122-58-3
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg

US $0.00/kg2025-08-21
- CAS:
- 1122-58-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons