Everolimus: An Review on the Mechanism of Action and Clinical Data
General Description
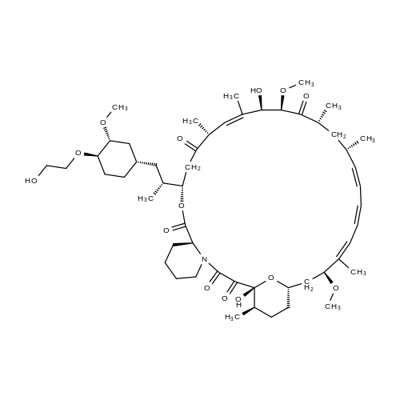
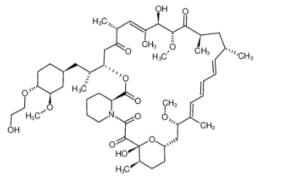
Everolimus [RAD001, Afinitor (40-O-(2-hydroxyethyl)-rapamycin)] is a derivative of rapamycin (sirolimus). It is an orally available selective inhibitor of mTOR. Like Rapamycin, it binds FKBP12 and inhibits the mTORC1 complex, abrogating downstream signaling of this pathway. mTORC1 is a downstream signal transducer of the PI3K pathway, which is frequently activated in human malignancies. Everolimus, like rapamycin, does not affect the activity of mTORC2 complex. Based on its mechanism of action, everolimus is not expected induce rapid cell death but rather to slow tumor growth.

Figure 1. Properties of Everolimus
Preclinical Data
Everolimus and other rapalogs inhibit the proliferation of various human tumor cell lines and human umbilical vein endothelial cells in vitro. The IC50 (dose at which growth is inhibited by 50 %) ranges from sub-nanomolar to micromolar, depending on the cell type. In vitro everolimus reduces expression of HIF1 and VEGF, suggesting that everolimus may also act as an anti-angiogenic agent. This anti-angiogenic activity of everolimus was confirmed in vivo. Mice with primary and metastatic tumors treated with everolimus showed a significant reduction in blood vessel density when compared to controls. The pharmacokinetic profile of everolimus in rats and mice showed sufficient tumor penetration, above what was needed to inhibit the proliferation of endothelial cells and tumor cell lines in vitro, and below concentrations reached in humans. Everolimus administered daily p.o. potently inhibited tumor growth in multiple different mouse and rat xenograft models.1
Indication
Gastric Cancer
Based on results from few smaller phase II trials, which had shown limited activity of everolimus; GRANITE-1 (NCT00879333) was designed. Results of this phase III trial in previously treated patients with advanced gastric cancer were presented at the ASCO Gastrointestinal Cancers Symposium 2012. In this trial, 656 patients were randomized 2:1 to receive everolimus (10 mg/day) plus BSC or placebo plus BSC. Baseline characteristics were well balanced. The primary endpoint, prolongation of OS, was not reached. Median OS was 5.4 months with everolimus versus 4.3 months with placebo (HR 0.90; 95 % CI 0.75–1.08; p = 0.124). Secondary endpoints included PFS and ORR. Median PFS per local assessment was 1.7 versus 1.4 months with PBO (HR 0.66; 95 % CI 0.56–0.78; p<0.0001).2
Liver Cancer
Preclinical evidence for a possible role of mTOR in HCC came from xenograft models, in which everolimus suppressed xenograft growth, provided the rationale for investigation of everolimus in HCC. One phase I/II trial in 28 patients with HCC determined 10 mg/day as recommended Everolimus 381 dose for phase II. Although possible clinical activity was noted, the trial did not reach its phase II stage. One phase III study in HCC compared the efficacy of everolimus (10 mg/day) versus placebo (EVOLVE-1). In this trial, 546 patients with HCC after failure of sorafenib were randomized (2:1) to receive everolimus 7.5 mg/day or placebo. The primary endpoint of prolongation of overall survival was not met.3
Lymphoma
Preclinical results showed increased sensitivity of everolimus-treated diffuse large B-cell lymphoma (DLBCL) cells to rituximab in vitro, and an increased cytotoxic effect when combined with other agents in mantle cell lymphoma (MCL), and in other models. Everolimus showed promising clinical activity as single agent in heavily pretreated Hodgkin lymphoma (HL). Of nineteen patients treated with everolimus (10 mg/day), eight patients achieved a PR and one patient achieved a CR. Median time to progression was 7.2 months.4
Neuroendocrine tumors
Based on the clinical trial studies, everolimus was approved in 2011 by the FDA for the treatment of progressive neuroendocrine tumors of pancreatic origin (PNET) in patients with unresectable, locally advanced or metastatic disease and by the EMA for the treatment of unresectable or metastatic, well- or moderately differentiated neuroendocrine tumors (NET) of pancreatic origin in adults with progressive disease.5
Pharmacokinetics and Pharmacodynamics
In a dose-escalation study of everolimus in 92 patients with advanced cancer patients, everolimus was rapidly absorbed after oral administration, with a median time to peak blood levels (tmax) of 1–2 hours after administration. Maximum tolerated dose (MTD) was not reached. The blood concentration was dose proportional over the dose range tested while maximum blood concentration Cmax appeared to plateau at dose levels higher than 20 mg/week. The terminal half-life was 30 h (range, 26–38 h) similar to that in healthy volunteers. Inter-patient variability was moderate. High-fat meals alter the absorption of everolimus. Everolimus is metabolized and excreted into the feces >80 %. Pharmacodynamic modeling based on S6 kinase inhibition in peripheral blood mononuclear cells suggested 5–10 mg daily to be an adequate dose to produce a high degree of sustained target inhibition.6
Absorption and Metabolism
In patients with advanced solid tumors, peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses ranging from 5 mg to 70 mg. Following single doses, Cmax is dose-proportional between 5 mg and 10 mg. At doses of 20 mg and higher, the increase in Cmax is less than dose-proportional, however AUC shows dose-proportionality over the 5 mg to 70 mg dose range. Steady-state was achieved within 2 weeks following once-daily dosing. Dose Proportionality in Patients with SEGA (subependymal giant-cell astrocytomas) and TSC (tuberous sclerosis complex): In patients with SEGA and TSC, everolimus Cmin was approximately dose-proportional within the dose range from 1.35 mg/m2 to 14.4 mg/m2.7
Everolimus is a substrate of CYP3A4 and PgP (phosphoglycolate phosphatase). Three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidylcholine conjugate of everolimus were the 6 primary metabolites detected in human blood. In vitro, everolimus competitively inhibited the metabolism of CYP3A4 and was a mixed inhibitor of the CYP2D6 substrate dextromethorphan.7
Toxicity
Everolimus has been investigated in over 30,000 patients in clinical studies and in post-marketing experience. In cancer patients, the main adverse events reported with everolimus were: stomatitis, non-infectious pneumonitis, infections, and renal failure. In addition, laboratory abnormalities, mainly hyperglycemia, hyperlipidemia, anemia, neutropenia, and thrombocytopenia were reported. For a recent and complete list of adverse drug reactions, please refer to your local drug label or package insert.
Adverse Effects
The most frequent adverse events of everolimus are hypercholesterolemia and hypertriglyceridemia. In one study comparing the side effects of sirolimus and everolimus in blood lipid profiles and in haematological parameters, the authors found greater hypercholesterolemia and hypertriglyceridemia in patients treated with sirolimus. Whether these high cholesterol and triglycerides levels are dangerous is unclear because of the antiatherosclerotic effects of these drugs. Everolimus was also shown to strongly inhibit atherosclerosis in LDL-receptor (-/-) mice despite severe hypercholesterolemia.8
Drug Interactions
Everolimus is mainly metabolized by CYP3A4 in the liver and to some extent in the intestinal wall. Everolimus is also a substrate of P-glycoprotein (PGP). Therefore, absorption and subsequent elimination of systematically absorbed everolimus may be influenced by medications that interact with CYP3A4 and/or PGP. In vitro studies showed that everolimus is a competitive inhibitor of CYP3A4 and of CYP2D6 substrates, potentially increasing the concentrations of medicinal products eliminated by these enzymes. Strong inhibitors of CYP3A4 (azoles, antifungals, cyclosporine, erythromycin) have been shown to reduce the clearance of everolimus therapy, thereby increasing everolimus blood levels. Similarly, Rifampin, a strong inducer of CYP3A4, increases the clearance of everolimus thereby reducing everolimus blood levels. Caution should be exercised when co-administering everolimus with CYP3A4 inhibitors or inducers.9
Preparation
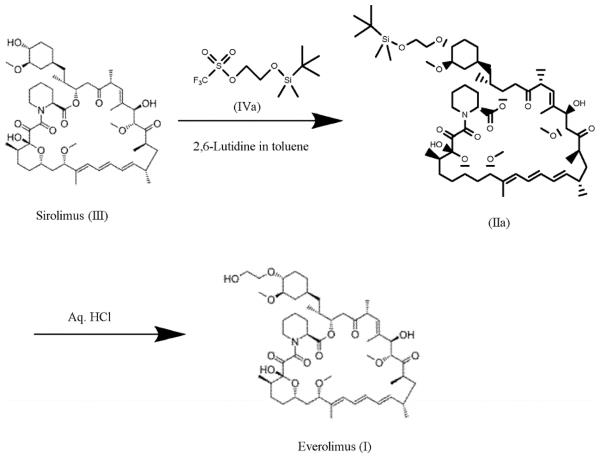
Figure 2. Preparation of Everolimus
For the synthesis, firstly sirolimus of formula (III) and 2-(t-butyldimethylsilyl)oxyethyl triflate of formula (IVA) are reacted in the presence of 2,6-Lutidine in toluene at around 60°C to obtain the corresponding 40-O-[2-(t-butyldimethylsilyl)oxy]ethyl rapamycin of formula (lla), which is then deprotected in aqueous hydrochloric acid and converted into crude everolimus [40-O-(2-Hydroxy)ethyl rapamycin] of formula (I).10
References
1. Lane HA et al (2009) mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res: Official J Am Assoc Cancer Res 15(5):1612–1622.
2. Yoon DH et al (2012) Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum. Br J Cancer 106(6):1039–1044.
3. Villanueva A et al (2008) Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 135(6):1972–1983.
4. Johnston PB et al (2010) A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol 85(5):320–324.
5. Pavel ME et al (2011) Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 378(9808):2005–2012.
6. O'Donnell A et al (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol: Official J Am Soc Clin Oncol 26(10):1588–1595.
7. Neumayer HH, Paradis K, Korn A, et al. Entry-into-human study with the novel immunosuppressant SDZ RAD in stable renal transplant recipients. Br J Clin Pharmacol 1999 ; 48 : 694 -703.
8. Mueller MA, Beutner F, Teupser D, et al. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR(-/-) mice despite severe hypercholesterolemia. Atherosclerosis 2007.
9. Kovarik JM, Beyer D, Schmouder RL. Everolimus drug interactions: application of a classification system for clinical decision making. Biopharm Drug Dispos 2006 ; 27 : 421-6.
10.WO2016020664A1
You may like
Related articles And Qustion
See also
Lastest Price from Everolimus manufacturers

US $0.00-0.00/kg2025-09-04
- CAS:
- 159351-69-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/g2025-07-11
- CAS:
- 159351-69-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 20kg