What is Everolimus?
Feb 13,2020
Everolimus is an orally administered, targeted therapy indicated for the treatment of advanced renal cell carcinoma. It inhibits the mammalian target of rapamycin, an integral component of multiple pathways involved in cell growth and proliferation.[1]
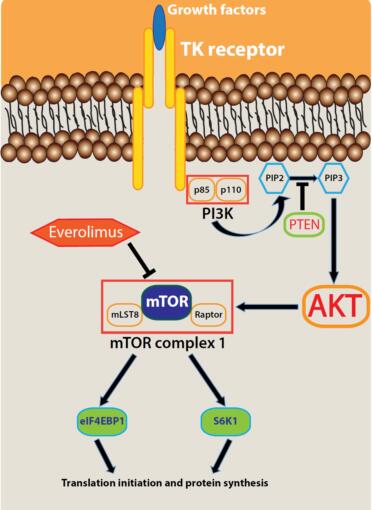
Everolimus is an orally bioavailable derivative of rapamycin (sirolimus), exerting its effect by entering the cell and binding to the immunophilin FK506 tacrolimus)-binding protein 12 Da isoform (FKBP12), forming a complex that binds to the FKBP-rapamycin-binding domain of mTOR, when that protein is part of a multiprotein complex (mTOR complex 1), thus inhibiting downstream signalling events. Everolimus is associated with cell growth retardation. Phosphorylation of the translational repressor eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) was reduced and the translational activator ribosomal protein S6 kinase 1 (S6K1) showed reduced activity in tumour, skin and peripheral blood mononuclear cell (PBMC) extracts when CA20948 pancreatic tumourbearing rats were administered everolimus. The antitumour activity of everolimus treatment was correlated with the prolonged inactivation of S6K1 in PBMCs in CA20948 pancreatic tumour-bearing rats; a prolonged inactivation of S6K1 was also observed in tumours and skin. [2]
Oral everolimus is absorbed rapidly, and reaches peak concentration after 1.3–1.8 hours. Steady state is reached within 7 days, and steady-state peak and trough concentrations, and area under the concentration-time curve (AUC), are proportional to dosage. In adults, everolimus pharmacokinetic characteristics do not differ according to age, weight or sex, but bodyweight-adjusted dosages are necessary in children. [3]
The advantage of everolimus is its lower nephrotoxicity in comparison with the standard immunosuppressants ciclosporin and tacrolimus. Observed adverse effects with everolimus include hypertriglyceridaemia, hypercholesterolaemia, opportunistic infections, thrombocytopenia and leukocytopenia. Everolimus is generally very well tolerated with most common side effects including stomatitis, rash, fatigue, hyperglycemia, hyperlipidemia, and myelosuppression. Most of these side effects are mild and resolve with dose interruptions or dose reductions. [4]
On July 20, 2012, the U.S. Food and Drug Administration (FDA) approved everolimus in combination with the steroidal aromatase inhibitor, exemestane, for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer, who experience recurrence or progression following treatment with a non-steroidal aromatase inhibitor.

References
1.Bellmunt J, Guix M. The medical management of metastatic renal cell carcinoma: integrating new guidelines and recommendations[J]. BJU Int 2009, 103 (5): 572-7
2.Kroog GS, Motzer RJ. Systemic therapy for metastatic renal cell carcinoma[J]. UrolClin NorthAm, 2008, 35 (4): 687-701
3.Bellmunt J. Future developments in renal cell carcinoma[J]. Ann Oncol 2009, 1: 13-17
4.Escudier B. Signaling inhibitors in metastatic renal cell carcinoma[J]. Cancer J 2008, 14 (5): 325-
You may like
Related articles And Qustion
Lastest Price from Everolimus manufacturers
Everolimus

US $0.00-0.00/kg2025-09-04
- CAS:
- 159351-69-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
Everolimus

US $0.00-0.00/g2025-07-11
- CAS:
- 159351-69-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 20kg