3,4,5-trichloroaniline- Reaction / Application on synthetic works
The synonyms of 3,4,5-trichloroaniline are 3,4,5-trichloroaniline, 3,4,5-trichloro aniline, 3,4,5-Trichloroaniline, 3,4,5-trichloro-aniline, 3,4,5-Trichlor-anilin, and 3,4,5-trichloro-benzenamine.
3,4,5-Trichloroaniline is an important organic intermediate to synthetize substituted benzene products.
Example 1
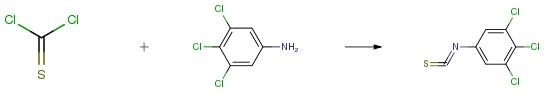
To a suspension of calcium carbonate (1.27 g, 12.7 mmol, Eq: 2.5) and thiophosgene (750 mg, 500 μ, 6.52 mmol, Eq: 1.28) in dichloromethane (13.2 g, 10.0 ml, 155 mmol, Eq: 25.3)/water (10.0 g, 10.0 ml, 555 mmol, Eq: 90.2) at 0, was added 3,4,5-trichloroaniline (1 g, 5.09 mmol, Eq: 1.00) The reaction was gradually warmed to room temperature and stirred overnight. Added 13 mL I M HC1. Separated organic layer and dried over sodium sulfate to give 1.12 g (92 percent) of desired product as an off-white solid.
Example 2
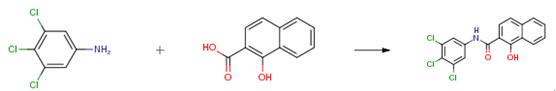
1-Hydroxynaphthalene-2-carboxylic acid (5.3 mmol) and the corresponding substituted aniline (5.3 mmol) were suspended in 30 mL of dry chlorobenzene. Phosphorous trichloride (2.65 mmol) was added dropwise, and the reacting mixture was heated in the microwave reactor at maximal allowed power 500 W and 130 °C, using infrared flask-surface control of temperature, for 15 min. The solvent was evaporated under reduced pressure, the solid residue washed with 2 M HCl, and the crude product was recrystallized from aqueous ethanol.
Example 3
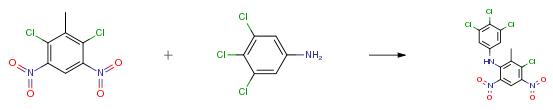
Substituted/unsubstituted aniline 6 (1equiv) was added in portions to a suspension of NaH (60 percent, 2 eq) and tetrahydrofuran (THF) at room temperature, the mixture was stirred for 30 min. 2,6-Dichloro-3,5-dinitrotulune (1.2equiv) in THF was added within 30 min, then stirred for another 5 h. After the reaction was over by thin-layer chromatography (TLC) monitoring, the reaction mixture was filtered. The filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether=1:20) to give the target compounds. Orange solid; mp. 163-164 °C; yield 75 percent.
Example 4
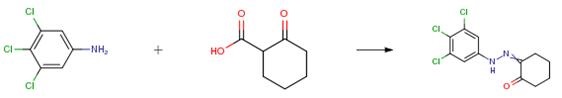
3,4,5-Trichloroaniline (1 ,6 g, 11.2 mmol) was dissolved in water (4 mL), cooled to 0 °C, cone. HC1 (1.5 ml) followed by a solution of NaNO2 (0.7 g, 11.2 mmol) in water (4 mL), was added dropwise and the resulting mixture was stirred for 30 min. followed by the addition of a solution of 2-oxocyclohexanecarboxylic acid. The reaction mixture was warmed to room temperature and stirred for 1 h during which time a solid precipitated out which was filtered and air dried to give the title compound (1.0 g, 29 percent).
References
1. F. Hoffmann-La Roche Ag, Hoffmann-La Roche Inc. Bilotta JA, Chen Z, Chin E, Ding Q, Erickson SD. Plancher JM, Weikert RJ. Triazole compounds as antivirals. WO2014/6066[P], 2014, A1, Paragraph 0168.
2. Gonec T, Kos J, Pesko M, Dohanosova J, Oravec M, Liptaj T, Kralova K, Jampilek J. Halogenated 1-Hydroxynaphthalene-2-Carboxanilides affecting photosynthetic electron transport in photosystem I[J]. Molecules, 2017, 22(10):22101709.
3. Li H, Guan A, Huang G, Liu CL, Li Z, Xie Y, Lan J. Design, synthesis and structure-activity relationship of novel diphenylamine derivatives[J]. Bioorganic and Medicinal Chemistry, 2016, 24(3):453 - 461.
4. Radius Health, Inc. Miller CP. Selective androgen receptor modulators. WO2011/97496[P], 2011, A1, Page column 78-79.
You may like
See also
Lastest Price from 3,4,5-TRICHLOROANILINE manufacturers
US $0.00/KG2022-01-15
- CAS:
- 634-91-3
- Min. Order:
- 1KG
- Purity:
- 97.3%
- Supply Ability:
- 100 tons