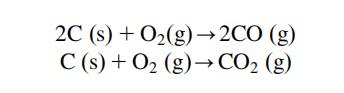Health effects of carbon
Carbon is unique in its chemical properties because it forms a number of components superior than the total addition of all the other elements in combination with each other.
Elemental carbon exists in two well-defined allotropic crystalline forms: diamond and graphite. Other forms with little crystallinity are vegetal carbon and black fume. Chemically pure carbon can be prepared by termic decomposition of sugar (sucrose) in absence of air. The physical and chemical properties of carbon depend on the crystalline structure of the element.

Application
The free element has a lot of uses, including decoration purposes of diamonds in jewelry or black fume pigment in automobile’s rims and printer’s ink. Another carbon form, the graphite, is used for high temperature crucibles, dry cell and light arch electrodes, for pencil tips and as a lubricant. Vegetal carbon, an amorphous form of carbon, is used as gas absorbent and bleaching agent.
Carbon compounds have plenty of uses. Carbon dioxide is used in drinks carbonatation, in fire extinguishers and, in solid state, as a cooler (dry ice). Carbon monoxide is used as reduction agent in many metallurgic processes. Carbon tetrachloride and carbon disulphide are important industrial solvents. Freon is used in cooling systems. Calcium carbide is used to prepare acetylene; it’s used for welding and cutting metals, as well as for preparation of other organic compounds. Other metallic carbides have important uses as heat-resistants and metal cutters.
Health effects of carbon
Elemental carbon is of very low toxicity. Health hazard data presented here is based on exposures to carbon black, not elemental carbon. Chronic inhalation exposure to carbon black may result in temporary or permanent damage to lungs and heart.Pneumoconiosis has been found in workers engaged in the production of carbon black. Skin conditions such as inflammation of the hair follicles, and oral mucosal lesions have also been reported from skin exposure.
Carcinogenicity- Carbon black has been listed by the International Agency for Research on Cancer (IARC) within Group 3 (The agent is not classifiable as to its carcinogenicity to humans). Some simple carbon compound can be very toxic, such as carbon monoxide (CO) or cyanide (CN-).
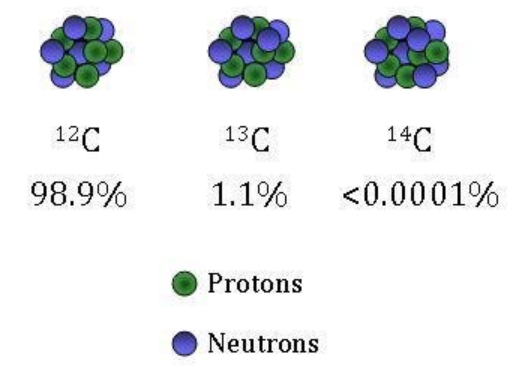
Carbon 14 is one of the radionuclides involved in atmospheric testing of nuclear weapons, which began in 1945, with a US test, and ended in 1980 with a Chinese test. It is among the long-lived radionuclides that have produced and will continue to produce increased cancers risk for decades and centuries to come. It also can cross the placenta, become organically bound in developing cells and hence endanger fetuses.
Most we eat is made up of compounds of carbon, giving a total carbon intake og 300 g/day. Digestion consist of breaking these compounds down into molecules than can be adsorbed to the wall of the stomach or intestine. There they are trasported by the blood to sites where they are utilized or oxidised to release the energy they contain.
Related articles And Qustion
See also
Lastest Price from Carbon manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7440-44-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $3.50/kg2025-04-18
- CAS:
- 7440-44-0
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 300tons/month