2-C-Methyl-D-ribono-1,4-lactone - Reaction / Application on synthetic works
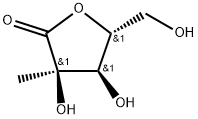
The synonyms of 2-C-Methyl-D-ribono-1,4-lactone are 2-C-methyl-D-ribono-1,4- lactone, (3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl) -3-methyldihydrofuran -2(3H)-one, 2-C-methyl-D-ribonic-γ-lactone, 2-C-methyl-D-ribono-γ-lactone, 2-C-methylribono-γ-lactone, 2-methylribonolactone, and 2,3-O-isopropylidene-2 -C-methyl-D-ribonic-gamma-lactone.
2-C-Methyl-D-ribono-1,4-lactone has been used in the synthesis of enantiomerically pure 4-substituted riboses and been also used for preparing saccharinic acids and lactones via Amadori rearrangement and for use as synthons toward herbicidal esters and branched nucleosides.
Example 1
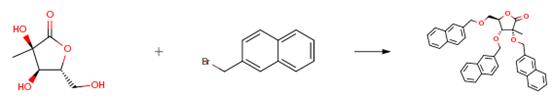
The raw material (5.0 g, 30.84 mmol) dissolved in anhydrous DMF (200 ml) in, nitrogen protection, cooling to -10 °C, slowly added 60% NaH (1.6 g, 40.09 mmol), in -10 °C stirring reaction for 1 hour later, slowly adding 2 - bromo naphthalene (10.2 g, 46 . 26 mmol), continuing to stir 30 minutes later, according to the above-mentioned operation by adding 60% NaH (1.6 g, 40.09 mmol) and 2 - bromo naphthalene (10.2 g, 46.26 mmol), repeated two, keeping the temperature at -10 °C stirring reaction 48 hours later, the reaction solution is poured into a 500 ml ice water quenching of the reaction, ethyl acetate (100 mL× 4) extraction, the combined organic phase, 200 ml saturated salt water washing of the organic layer, anhydrous sodium sulfate drying, concentrating, separating by silica gel column (10:1, V/V, petroleum ether: EtOAc) to obtain colorless oily product (12.6 g, 21 . 59 mmol), yield: 70%.
Example 2

The 2-C-methyl-D- ribotide -1,4-lactone (1.62g, 10mmoL) suspended in 40 ml of ethyl acetate, under the condition of ice bath, by adding 4-chloro-benzoyl chloride (3.48g, 20mmoL, 2eq), slowly add triethylamine (3.0 ml, 22mmoL, 2.2eq), 2 hours to drop end, stirring overnight. Filtering, with 20 ml ethyl acetate wash the filter cake, combined with the phase, with saturated sodium bicarbonate, 1M dilute hydrochloric acid and saturated salt water washing, drying by anhydrous magnesium sulphate. Concentrated after filtering, column separation to obtain 2.45g white solid, yield 56 percent.
Example 3

To a flask was charged NaH (1.7 g) and N,N-dimethylfonnamide (30 mL). The solution was cooled in an ice bath and Lactone A (1.57 g) was added in DMF (4 mL) followed by a wash with DMF (1 mL). The ice bath was removed and after 1.5 h the reaction mixture was cooled in an ice bath and 3,3-dimethylallyl bromide (5.2 mL) was added. The ice bath removed and the reaction left to stir overnight. The reaction mixture was cooled to 0 °C and was quenched with saturated NH4C1 (3 mL) followed by diluting with water (27 mL) and EtOAc (100 mL). The organics were then washed with water and brine (30 mL each) and then dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel giving 1.42 g (40percent) of the tri-prenyl Lactone G.
Example 4

To a stirred suspension of starting material (300 g, 1.86 mol) in acetone (4 L) was added conc.H2SO4 (56 mL) dropwise at RT. The mixture was stirred at RT for 3 h. The mixture was neutralized with solid NaHCO3 and filtered. The filtrate was evaporated under reduced pressure to give 287-2 (381 g, crude) as a colorless oil, which was used for the next step without further purification.
References
1. Chinese Academy of Sciences Shanghai Pharmaceutical Institute. Shen J, Li J. Chen Y, Zhou Y, Xiong B, Liu T, Wang Y, Xu W. 2' - C - methyl substituted nucleoside compound and its preparation and use (by machine translation). CN109748943[P], 2019, A, Paragraph 02888-0292.
2. Suzhou Wangshan Wangshui Biological Pharmaceutical Co. Ltd. Topharman Shanghai Co. Ltd., Shanghai Institute of Materia Medica Chinese Academy of Sciences. Xie Y, Tian G, Jiang X. (2S, 3R, 4R) - 3,5-disubstituted-2-deoxy-2- hydroxy-2-methyl-D-ribose γ-the method for preparing lactone and intermediates (by machine translation). CN105693661[P], 2016, A, Paragraph 0128; 0129; 0130; 0131.
3. Gilead Sciences, Inc. Butler, T, Cho A, Graetz BR. Kim CU, Metobo SE, Saunders OL, Waltman AW, Xu J, Zhang L. Processes and intermediates for the preparation of 1'-substituted carba-nucleoside analogs. WO2011/35250[P], 2011, A1, Page column 82.
4. Alios Biopharma Inc. Blatt LM, Beigelman L, Dyatkina N, Symons JA, Smith DB. Substituted nucleosides, nucleotides and analogs thereof. US2015/366887[P], 2015, A1. Paragraph 1074-1075.
You may like
Related articles And Qustion
See also
Lastest Price from 2-C-Methyl-D-ribono-1,4-lactone manufacturers

US $0.00/KG2025-04-21
- CAS:
- 492-30-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 500kg/month

US $10.00/KG2025-04-21
- CAS:
- 492-30-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons