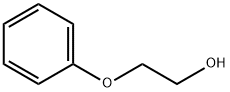
2-phenoxyethanol in vaccines
Introduction
2-Phenoxyethanol, known early on as ethylene glycol monophenyl ether, is a high-boiling, oily liquid that is moderately water-soluble. It has a long history and several uses in chemical manufacturing and consumer products. The earliest mention of 2-phenoxyethanol in the chemical literature appeared in 1894 when Ernst Roithner at the University of Vienna published a treatise on ethylene oxide. In the article, Roithner described the synthesis of 2-phenoxyethanol via the reaction of ethylene oxide with phenol in a basic medium.
Phenoxyethanol, or 2-phenoxyethanol, has a large spectrum of antimicrobial activity and has been widely used as a preservative in cosmetic products for decades. It is effective against various Gram-negative and Gram-positive bacteria, as well as against yeasts, and has only a weak inhibitory effect on resident skin flora[1].
Preservatives in vaccines
There is a safety concern about several vaccine preservatives today, such as formaldehyde, phenol, organic mercury (thiomersal), or betapropiolactone, for safety reasons. In a few vaccines, 2-phenoxyethanol has been used as a vaccine perservative, but has not gained widespread use because the preservative effect is inferior to, e.g., thiomersal.
Phenol is used in various consumer products, including mouthwashes, throat lozenges, and throat sprays. It is also currently used as a preservative in three FDA-approved available vaccines, Pneumovax 23 (for prevention of pneumococcal disease caused by the 23 serotypes contained in the vaccine) and Typhim Vi (for prevention of typhoid fever) and ACAM2000 (for prevention of smallpox); each of these vaccines contains 0.25% phenol. These vaccines are not recommended for routine use by the Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices (ACIP).
2-Phenoxyethanol is an organic chemical compound sometimes used in cosmetics and antiseptics. It is also currently used as a preservative in one FDA-approved available vaccine, Ipol, for the prevention of polio at a concentration of 0.5%.
Thimerosal is a mercury-containing organic compound (an organomercurial). Since the 1930s, it has been widely used as a preservative in several biological and drug products, including many vaccines, to help prevent potentially life-threatening contamination with harmful microbes. The documented antimicrobial properties of Thimerosal contribute to the safe use of vaccines in multi-dose vials, and the ability to package certain vaccines, such as those for seasonal and pandemic influenza, in multi-dose vials helps facilitate immunization campaigns in the United States and globally that save lives. However, the use of Thimerosal as a preservative in U.S. FDA-licensed vaccines has significantly declined due to the reformulation and development of new vaccines presented in single-dose containers.
The efficacy of two antimicrobial preservatives,2-phenoxyethanol (2-PE) and Thimerosal, was compared in adsorbed diphtheria-purified pertussis-teta-nus combined vaccine (DPT vaccine). Thimerosal had strong microbicidal activity against Gram-positive bacteria, Gram-negative bacteria, yeast, and fungi at 25C and 4°C. 2-PE also had microbicidal activity against these microorganisms but only had microbistatic activity against fungi at 4°C. Neither 2-PE nor Thimerosal affected the potency or toxicity of the DPT vaccine. These data suggest that 2-PE has weaker antimicrobial activity than Thimerosal against yeast and fungi in the DPT vaccine at low temperatures[2].
Using human neuroblastoma cells, the relative cytotoxicity of the levels of the compounds commonly used as preservatives in US-licensed vaccines was found to be phenol<2-phenoxyethanol < benzethonium chloride < Thimerosal. The observed relative toxicity indices (human neuroblastoma cells/bacterial cells) were 2-phenoxyethanol (4.6-fold) < phenol (12.2-fold) < Thimerosal (>330-fold). In addition, for the compounds tested, except for 2-phenoxyethanol, the concentrations necessary to induce significant killing of bacterial cells were significantly higher than those routinely present in US-licensed vaccine/biological preparations[3].
References
[1] B. Dréno. “Safety review of phenoxyethanol when used as a preservative in cosmetics.” Journal of the European Academy of Dermatology and Venereology 33 S7 (2019): 15–24.
[2] Eiji Komatsu. “Influence of Temperature on the Efficacy of 2-Phenoxyethanol as a Preservative for Adsorbed Diphtheria-Purified Pertussis-Tetanus Combined Vaccine.” Journal of Health Science 132 1 (2002): 89–92.
[3] David A Geier, Mark R Geier, Sarah K Jordan. “The relative toxicity of compounds used as preservatives in vaccines and biologics.” Medical Science Monitor : International Medical Journal of Experimental and Clinical Research 16 5 (2010): SR21-7.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Phenoxyethanol manufacturers
US $0.00-0.00/KG2025-11-18
- CAS:
- 122-99-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00-0.00/kg2025-11-18
- CAS:
- 122-99-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg