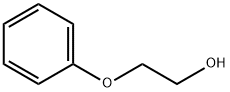
What is 2-Phenoxyethanol?
Description
2-Phenoxyethanol, known early as ethylene glycol monophenyl ether, is a high-boiling, oily liquid that is moderately water-soluble. At present, there has been a lot of research on the synthesis and use of this substance. Due to its excellent properties, it has been widely used in scientific research and industrial production[1].

Preparation
In 1894, Ernst Rosner of the University of Vienna published a paper on ethylene oxide. This is the earliest mention of 2-phenoxyethanol. He describes the synthesis of 2-phenoxyethanol via the reaction of ethylene oxide with phenol in a basic medium. 2-Phenoxyethanol is an organic compound and is an ethylene glycol ether. Its molecular formula is C8H10O2. Under the action of sodium acetate or sodium hydroxide, phenol and ethylene oxide condensation can synthesize 2-phenoxyethanol. The reaction needs to be carried out at a temperature of 20 °C and a pressure of 0.2-0.25 MPa.
Applications
2-Phenoxyethanol is very versatile and can be used as a reagent in organic chemical synthesis. It can also be used as a preservative for cosmetics and pharmaceuticals, a fixative for perfumes, a lubricant preservative, and an attractant for pesticides. In industrial production, it can be used as a solvent for dyes, inks, and resins[2-3]. 2-Phenoxyethanol can affect the low-temperature (95) staining of aramid. Through 2-phenoxyethanol pretreatment of meta-aramid fibres under different conditions, the study found that the meta-aramid fibre could be dyed more thoroughly with an appropriate concentration of 2-phenoxyethanol and a dramatic improvement in dyeing performance to aramid after 2-phenoxyethanol pretreatment[4]. 2-Phenoxyethanol (PhE) has been shown to induce hepatotoxicity, renal toxicity, and hemolysis in subchronic and chronic studies in multiple species. It is one of the endocrine-disrupting compounds, which are listed as priority emerging water contaminates. Therefore, it makes sense to remove 2-phenoxyethanol from water. Currently, studies are using ultrasonic irradiation at 600 kHz to remove 2-phenoxyethanol (PhE) from water[5].
References
[1] Witeska M, et al. Haematological effects of 2-phenoxyethanol and etomidate in carp (Cyprinus carpio L.). Veterinary Anaesthesia and Analgesia, 2016; 42: 537-546.
[2] Chen X, et al. Effect of 2-phenoxyethanol pre-treatment on structure and adhesion properties of thermotropic liquid crystal polyarylate fibers. Textile Research Journal, 2021; 91.
[3] Akbary P, et al. Analysis of primary and secondary stress responses in bighead carp ( Hypophthalmichthys nobilis ) by anesthetization with 2-phenoxyethanol. International Journal of Environmental Science and Technology, 2016; 13: 1009–1016.
[4] Sheng D, et al. Low-temperature dyeing of meta-aramid fabrics pretreated with 2-phenoxyethanol. Coloration Technology, 2017; 133: 320-324.
[5] Boutamine Z, et al. Sonochemical and photosonochemical degradation of endocrine disruptor 2-phenoxyethanol in aqueous media. Separation and Purification Technology, 2018; 206: 356-364.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Phenoxyethanol manufacturers
US $0.00-0.00/KG2025-11-18
- CAS:
- 122-99-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00-0.00/kg2025-11-18
- CAS:
- 122-99-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg