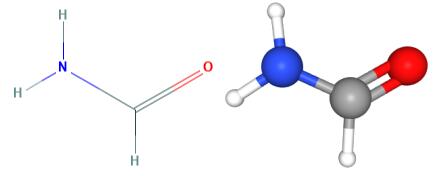
The structure of Formamide
Introduction
Formamide, the simplest carboxylic acid amide, is a viscous, odorless, colorless liquid with a melting point of 2 ºC and a boiling point of 210 ºC. It is an amide manufactured from methyl formate and ammonia. It is a clear liquid, miscible with water, ethanol, and acetone, and has a slight ammoniacal odor. It decomposes into ammonia, carbon dioxide, and water in the environment.
Formamide is a chemical reactant or solvent in various chemical processes. It is mainly used as a reactant in the manufacture of agrochemicals (e.g., imidazoles), pharmaceuticals (e.g., allopurinol), and industrial chemicals (e.g., n-vinylformamide) and as a solvent in polymers and resins. Formamide is registered with strictly controlled conditions as defined in Article 18(4) of Regulation (EC) No. 1907/2006 (REACH regulation) and must, therefore, be handled as such.
Structure
The formamide molecule contains a total of 6 atom(s). There are 3 Hydrogen atom(s), 1 Carbon atom(s), 1 Nitrogen atom(s), and 1 Oxygen atom(s). A chemical formula of formamide can, therefore, be written as CH3NO. The Lewis structure of formamide is shown below:
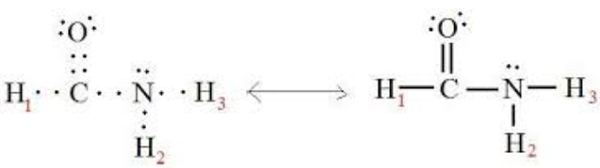
Formamide (HCONH2) also called methanamide. The molecular geometry is not linear; its shape is similar to water. The Lewis structure involves a double bond between carbon and oxygen, a single bond between carbon and hydrogen, a single bond between carbon and nitrogen, and a single bond between nitrogen and hydrogen. It is highly polar. Polar means unequal sharing of electrons between atoms. In formamide, the nitrogens appear to be sp3 hybridized, implying tetrahedral geometry. However, analysis shows that the molecule is very nearly planar, with bond angles close to 120 degrees.
You may like
Related articles And Qustion
See also
Lastest Price from Formamide manufacturers
US $0.00-0.00/KG2025-09-10
- CAS:
- 75-12-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20tons

US $0.00/KG2025-04-21
- CAS:
- 75-12-7
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 10tons/month