2-Methyl-4-isothiazolin-3-one: Uses and Toxicity
Uses of 2-Methyl-4-isothiazolin-3-one
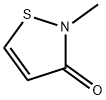
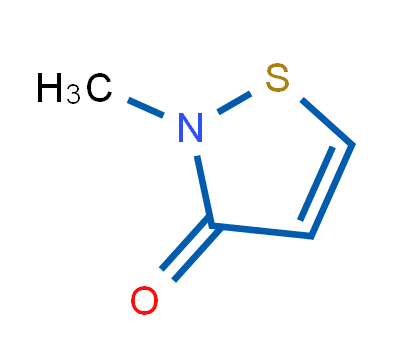
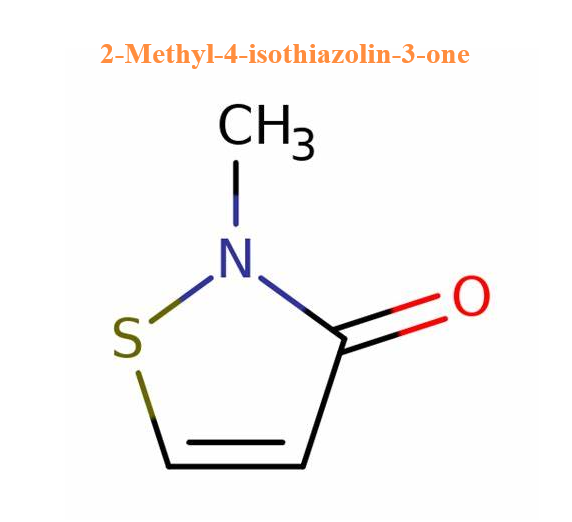
2-Methyl-4-isothiazolin-3-one, also known as Methylisothiazolinone, abbreviated as MIT, is a highly effective biocide and preservative widely used in cosmetics, personal care and industry. It is used as a biocide in cosmetics, hygiene products, textile production, pulp/paper mills, industrial processes, cooling water systems, oil field operations, and the production of air purifier systems; and as a preservative in adhesives, coatings, fuels, metalworking fluids, resin emulsions, paints, and wood products. Its structure is shown below:
It is a well-established preservative in personal care and cosmetics in combination with 5-chloro-2-methyl-4-isothiazolin-3-one (CIT), and the combination of CIT and MIT is also used as a wet industrial preservative and biocide. The combination of CIT and MIT is also used as a wet industrial preservative and biocide, and while CIT is the more effective biocide, it is also the least stable of the two components. Although MIT has relatively low antimicrobial properties on its own, it has been found that it does have synergistic activity in combination with BIT. For example, the individual Minimum Inhibitory Concentrations (MICs) of BIT and MIT against Pseudomonas aeruginosa are 150 ppm and 30 ppm, respectively, while the MIT/BIT combination has a MIC of 20 ppm.
MIT/CIT has a maximum use level of 15 ppm in personal care and cosmetics because it is a skin sensitiser and is therefore mainly used in rinse-off products. However, it is also very effective at very low concentrations, has broad-spectrum activity, is water-soluble and is compatible with non-ionic surfactants.
Toxicity of 2-Methyl-4-isothiazolin-3-one
In vitro studies have shown that brief exposure to MIT is highly toxic to cultured neurons but not to glial cells. The toxic effects of this fungicide are zinc-dependent and require activation of p44/42 extracellular signal-regulated kinase (ERK) via a 12-lipoxygenase-mediated pathway. The cell death process also includes activation of NADPH oxidase, generation of reactive oxygen species, DNA damage, and over-activation of poly (ADP-ribose) polymerase, all of which occur downstream of ERK phosphorylation. The Expert Panel on Cosmetic Ingredient Review noted evidence of in vitro neurotoxicity, but concluded that the absence of any neurotoxicity in numerous in vivo studies, including subchronic, chronic, and reproductive and developmental animal studies, suggests that MIT is not neurotoxic when applied to cosmetics.
However, MIT is a sensitiser in both animal and human studies. The Panel concluded that this was due to the existence of a threshold dose response and that concentrations of MIT of 100 ppm (0.01 per cent) or less in cosmetics were not expected to pose a sensitisation risk. Therefore, MIT can be safely used as a preservative in cosmetic products up to this concentration. Animal studies showed no treatment-related increase in tumour incidence in either male or female rats. In addition, MIT was not found to be foetotoxic, embryotoxic or teratogenic in rats.
References:
[1] GUILLAUME PELLETIER. Effects of a 28-day oral exposure to a 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one biocide formulation in Sprague-Dawley rats.[J]. Drug and Chemical Toxicology, 2014, 37 2. DOI:10.3109/01480545.2013.834353.You may like
Related articles And Qustion
See also
Lastest Price from 2-Methyl-4-isothiazolin-3-one manufacturers

US $0.00-0.00/KG2025-06-28
- CAS:
- 2682-20-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 2682-20-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt